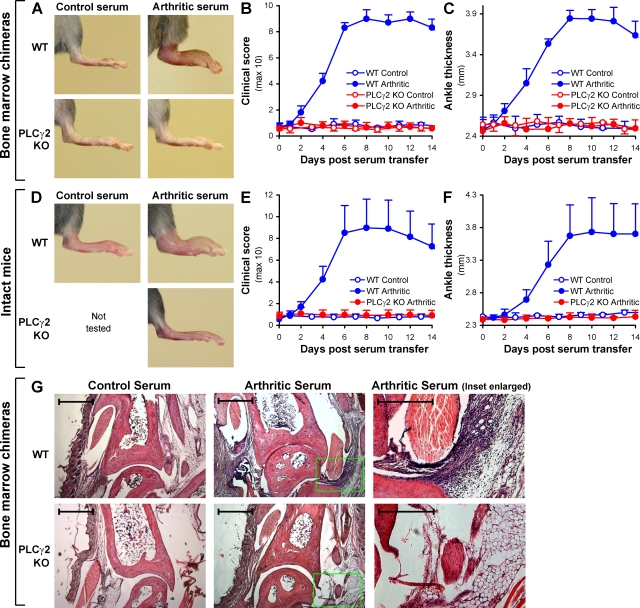
Figure 7.
PLCγ2 is required for the development of K/B×N serum transfer arthritis. WT and PLCγ2−/− (PLCγ2 KO) bone marrow chimeras (A–C and G) or intact (nonchimeric) mice (D–F) were injected with 400 µl of arthritic (K/B×N) or nonarthritic control serum and the development of arthritis followed. (A) Photographs of the hind limb of mice of the indicated treatment and hematopoietic genotype 10 d after serum injection. Pictures are representative of a total of 17–23 individual mice per group from eight independent experiments. (B and C) Hind limb clinical score (B) and ankle thickness (C) of mice of the indicated treatment and genotype. Error bars represent the SD of four to eight individual clinical scores or ankle thickness values from a single experiment repeated a total of eight times. (D–F) Hind limb photographs (D), clinical score (E), and ankle thickness (F) of intact (nonchimeric) mice of the indicated treatment and genotype. Data are from three mice per group tested in parallel. Error bars represent the SD of six individual hind limb values from three mice per group. (G) Histological analysis of the ankle joint of mice of the indicated treatment and hematopoietic genotype 4 d after serum injection. The photomicrographs on the right are enlarged from the highlighted areas in the middle pictures. Original magnification, 5×. Bars: (left and middle) 200 µm; (right) 100 µm. Photomicrographs are representative of a total of four to six samples per group from three independent experiments.