Figure 5.
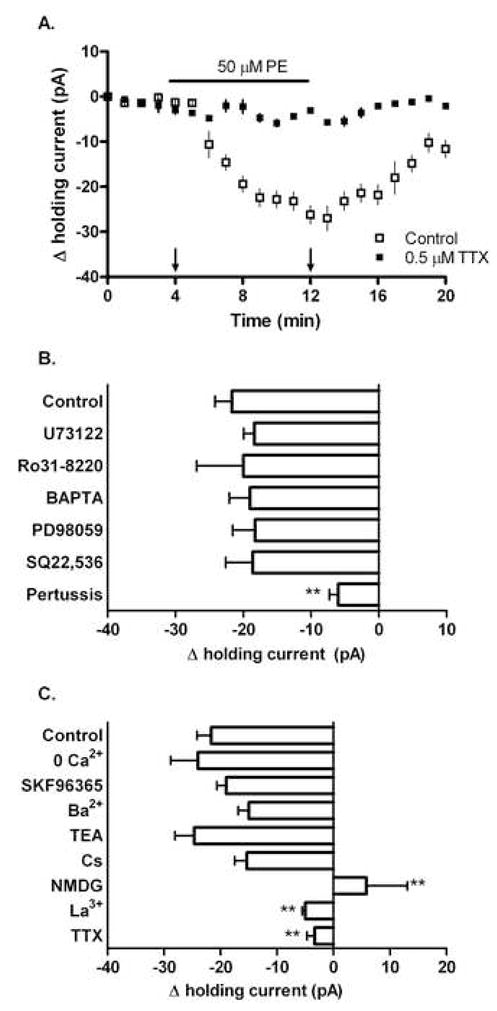
PE initiates a pertussis-sensitive inward sodium current in responsive CA1 interneurons. Whole-cell recordings of holding current in CA1 interneurons voltage clamped to −65 mV. In control aCSF, 50 μM PE produced a measurable inward current (−21±2.5 pA) in a subpopulation of CA1 interneurons. (A) Time course illustrating the PE-mediated inward current in control and TTX conditions. Arrows indicate time points used for Δ holding current calculations shown in (B) and (C). (B) The PE-mediated inward current was not significantly altered by pre-treatment with 50 μM U73122 (−18±1.6 pA), 100 nM Ro31-8220 (−20±6.9 pA), 10 mM BAPTA (−19±3.1 pA), 10 μM PD98059 (−18±3.3 pA), or 20 μM SQ22,536 (−18±3.9 pA). Pre-treatment with 100 μM pertussis toxin however significantly reduced the magnitude of the PE-mediated current (−6.0±1.3 pA). (C) Manipulations of Ca2+, Na+ and K+ availability differentially affected the observed PE-mediated inward current. In control aCSF, 50 μM PE produced a measurable inward current in a subpopulation of CA1 interneurons (−21±2.5 pA). This effect was not altered by removal of extracellular calcium from the aCSF (−24±4.8), or by pre-treatment with 100 μM SKF96365 (−19±1.7 pA), 2 mM barium (−15±1.8 pA), 10 μM TEA (−24±3.4 pA), or 3 mM cesium (−15±2.1 pA). However, significant reductions in the magnitude of the PE-mediated current were seen when NMDG was substituted for extracellular sodium (5.8±7.2 pA), or when slices were pre-treated with 10 μM lanthanum (−5.0±0.52 pA) or 0.5 μM TTX (−3.0±1.3 pA). For each experiment n=8.
