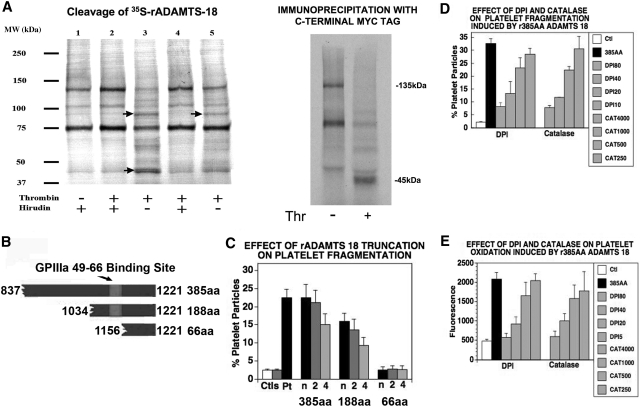
Figure 2.
Thrombin cleaves ADAMTS-18. (A) (Left panel) S35-methionine labeled ADAMTS-18 was incubated with different concentrations of thrombin with and without hirudin, at room temperature for 1 hour and resolved with SDS-PAGE. The sample loading lanes are (1) S35-ADAMTS-18 control (5 units/mL hirudin alone); (2) S35-ADAMTS-18 + 5 units/mL thrombin + hirudin; (3) S35-ADAMTS-18 + 5 units/mL thrombin; (4) S35-ADAMTS-18 + 2.5 units/mL thrombin + hirudin; (5) S35-ADAMTS-18 + 2.5 units/mL thrombin. Arrows point to thrombin cleaved bands. (Right panel) Immunoprecipitation with anti-Myc Ab following thrombin cleavage, demonstrating C-terminal 45-kDa fragment. (B) Graphic of truncated ADAMTS-18 constructs. The GPIIIa49-66 binding site is depicted. Three C-terminal recombinant peptide fragments were tested for their ability to induce platelet fragmentation and oxidation. These included the larger fragment of 385 amino acids (AAs) from the C-terminal end, an intermediate fragment of 188 AAs, and a smaller fragment of 66 AAs. (C) Effect of truncation on platelet fragmentation. Ctls refer to buffer and control IgG. Pt is patient IgG. Dilutions of the 3 fragments: recombinant peptides were made at neat (n), 1:2, and 1:4 in buffer. The initial concentration of the large 385-AA fragment was 1.3 μM, whereas the initial concentrations of the 188-AA and 66-AA peptides were 19 and 85 μM, respectively (n = 6). (D) Fragmentation induced by 385-AA fragment is inhibited by DPI (nM) and catalase (units/mL). Ctl refers to control buffer; 385-AA fragment refers to 1.3 μM 385-AA fragment. DPI 180, 140, 120, and 10 refer to nM DPI + 385-AA fragment. CAT 4000, 1000, and 500 refer to catalase units + 385-AA fragment. (E) Oxidation induced by 385-AA fragment inhibited by DPI and catalase. The designation is the same as that used as in panel D. (C-E) Where shown, error bars indicate SEM.