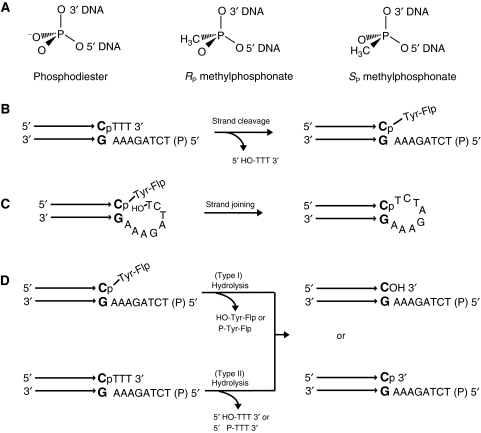
Figure 2.
Flp reactions using half-site substrates containing a phosphodiester or methylphosphonate at the scissile position. (A) The methyl substitutions in the RP and SP stereoisomers of methylphosphonate are indicated with reference to the achiral phosphodiester. (B) In the schematic representation of a half-site substrate, the horizontal arrows represent the Flp-binding element with its terminal bp abutting the spacer segment shown in bold. The scissile phosphodiester is indicated as ‘P'. Strand cleavage in a half-site traps the tyrosyl intermediate, as the trinucleotide product (5′HO-TTT3′) diffuses away. The 5′ end of the bottom strand is blocked by phosphorylation (denoted by ‘P' in parentheses) from acting as the nucleophile in a joining reaction. (C) A free 5′-OH group on the bottom strand permits the conversion of the tyrosyl intermediate into a hairpin recombinant. Although not shown here, attack by the 5′-OH from a second half-site is also possible. The joined strand would be identical in sequence to the hairpin. (D) Flp-assisted hydrolysis could potentially target the phosphotyrosyl intermediate formed by strand cleavage (top) or the scissile phosphodiester in DNA (bottom).The former activity is called type I endonuclease, the latter, type II.