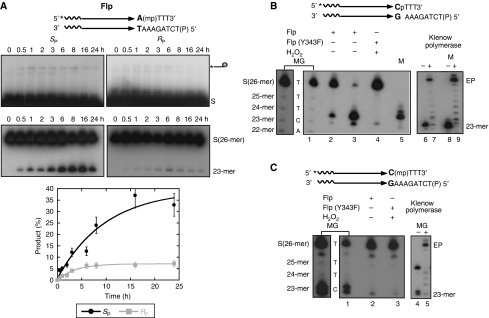
Figure 4.
Stereoisomer preference of Flp in the MeP-half-site reaction; characterization of the hydrolysis products from P- and MeP-half-site substrates. (A) The hydrolysis products from stereochemically pure SP and RP forms of the MeP-half-site were analysed by denaturing PAGE. (B) The closely migrating hydrolysis products from the P-half-site (2 and 24 h reactions in lanes 2 and 3, respectively, were characterized for the chemical nature of their 3′ ends. ‘MG' refers to a Maxam–Gilbert sequencing ladder obtained by chemical modification at C plus T positions followed by strand scission (lane 1). Lane 1 is duplicated to its left at higher intensity and contrast for better visibility of individual bands. Their sizes and the bases present at 3′-ends are indicated. A peroxidolysis reaction performed on the half-site using Flp(Y343F) was run in lane 4. ‘M' stands for a synthetic oligonucleotide marker harbouring a 5′-OH end. ‘EP' refers to the extension product formed in a Klenow polymerase reaction. (C) The hydrolysis product from a raecemic mixture of the MeP-half-site (lane 2) was characterized as in (B) by comparison to the C plus T sequencing ladder (lane 1 and its higher intensity duplicate to its left) and the product of Flp(Y343F)-assisted peroxydolysis (lane 3). The MeP substitution significantly enhanced strand scission to yield elevated levels of the 23-mer during the chemical steps of sequencing. The slow migrating band well above the doublet in lane 4 is the labelled top strand of the half-site, whose 3′-OH could prime DNA elongation by the Klenow enzyme.