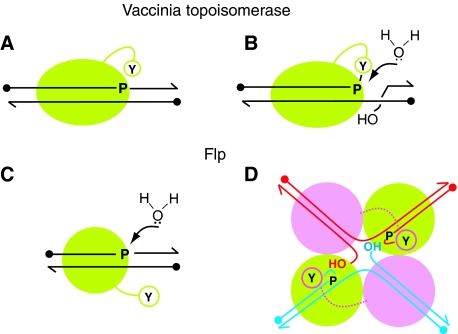
Figure 7.
Vulnerability of the transesterification steps of DNA relaxation and site-specific recombination to hydrolysis. (A) During DNA relaxation by type I topoisomerase, the strand cleavage step is protected by the engagement of the active site in cis and the in-line orientation of the active site tyrosine with respect to the scissile phosphate. (B) The tyrosyl intermediate resulting from strand cleavage is a potential target for attack by water during the strand rotation step that precedes ligation. Protection is derived from phosphate electrostatics. (C) The association of a single Flp monomer with DNA activates the scissile phosphate but full engagement of the active site must await the binding of a second Flp monomer and donation of the active site tyrosine. Hydrolysis of the phosphodiester bond is at potential risk. Active site electrostatics comes to the rescue. (D) During normal recombination, the synaptic architecture of the Flp tetramer, with the tight polypeptide interface between active pairs of Flp monomers, barricades water from dissipating the cleaved tyrosyl intermediate. Furthermore, the conformational dynamics accompanying cleavage orients the 5′-OH groups for strand joining.