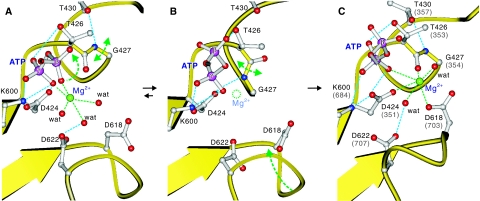
Figure 6.
A scenario for the removal of Mg2+ from ATP and re-binding to the P-domain before phosphoryl transfer. Models were built from the crystal structures of MolA (A) and MolB (B) of CopA-PN and the E1·AMPPCP form of Ca2+-ATPase (C), and the residue numbers refer to those of CopA; those of Ca2+-ATPase are shown in parentheses (C). Only the β- and γ-phosphates of ATP are shown. Lys425 to Leu429 form a π-helix (A, C), which is converted to a normal α-helix when Thr426-Gly427 peptide bond is flipped (B). Mg2+ bound to ATP (A) dissociates by the flipping (B) and binds to the P-domain bridging the γ-phosphate of ATP, the phosphorylation residue (D424) and a Mg2+-coordinating residue (D618). Broken lines show likely hydrogen bonds (cyan) and coordination of Mg2+ (green). Small spheres represent Mg2+ (green) and coordinating water molecules (red). Arrows indicate expected movements during the Mg2+ relocation.