Abstract
Study objective
HOXA10 is an essential regulator of endometrial receptivity. To determine the effect of gonadotropin releasing hormone (GnRH) antagonists on endometrial receptivity we assessed endometrial HOXA10 expression in GnRH antagonist, GnRH agonist, and natural cycles.
Design
Prospective case-control study
Setting
University academic medical center
Patients
Nineteen subjects were included: 12 subjects underwent controlled ovarian hyperstimulation (COH) with recombinant follicle stimulating hormone (rFSH) and used either a GnRH antagonist or a GnRH agonist; 7 control subjects underwent natural cycles.
Interventions
Pipelle endometrial biopsies were obtained 11 days after human chorionic gonadotropin (hCG) administration or spontaneous luteinizing hormone (LH) surge in untreated cycles, respectively. Immunohistochemistry was used to assess HOXA10 protein expression in endometrial glands and stroma.
Main outcome measure(s)
Endometrial HOXA10 protein expression
Results
HOXA10 expression was significantly decreased in endometrial stromal cells in GnRH antagonist treated cycles compared with GnRH agonist treated cycles or natural cycle controls. There was no significant difference in glandular cell HOXA10 expression among the three groups.
Conclusions
Use of GnRH antagonists may be associated with impaired HOXA10 expression in endometrial stromal cells, and thus may affect endometrial receptivity.
KEY WORDS: endometrial receptivity, GnRH antagonist, GnRH agonist, HOXA10, implantation
Introduction
Controlled ovarian hyperstimulation (COH) with recombinant follicle stimulating hormone (rFSH) is commonly used in the treatment of infertility. Gonadotropin releasing hormone (GnRH) analogs, both agonists and antagonists, are often employed to prevent a premature luteinizing hormone (LH) surge and subsequent ovulation. While a regimen of these medications can result in increased oocyte production, implantation rates remain relatively low; the majority of human embryos fail to implant (1,2). Endometrial receptivity is an essential part of implantation success, and it is crucial to determine the effect of these medications on the endometrium.
The effect of COH on implantation remains controversial (3–7). FSH and GnRH receptors have been identified in the endometrium, thus it is possible that gonadotropins and GnRH analogues may have a direct or indirect effect on the endometrium (8–10). Furthermore, high serum estradiol levels or other hormonal alterations that result from FSH stimulation may adversely affect the endometrium (5,7,11,12).
The effects of GnRH agonists and GnRH antagonists on endometrium and pregnancy rates have previously been investigated. Both medications are associated with advanced endometrial maturation of 2 to 4 days on the day of oocyte retrieval; no pregnancies occur when the advancement is greater than 3 days (13–15). However recently, endometrial development during cycles using GnRH antagonists were found to be histologically more similar to endometrium from natural cycles than endometrium exposed to GnRH agonists (16). Although most studies do not identify significant differences in clinical pregnancy rates with the use of GnRH antagonists (17,18), or with GnRH antagonists compared with GnRH agonists (19–21), several reviews and meta-analyses report an overall decrease in pregnancy rates with the use of GnRH antagonists compared with GnRH agonists (21–28).
Here we investigated the effect of GnRH antagonists on HOXA10, a well-characterized marker of endometrial receptivity (29,30). HOXA10 is a homeobox-containing transcription factor that regulates uterine development in the embryo as well as adult endometrial development during each menstrual cycle (29–33). HOXA10 expression is necessary for endometrial receptivity (30,33–36). Targeted mutation of HOXA10 renders mice infertile due to implantation failure: they produce viable embryos, and these embryos implant and develop normally in a wild-type surrogate, however wild-type embryos fail to implant in HOXA10 (−/−) mice (37). This phenotype is likely related to both the absence of HOXA10 during embryonic uterine development, and lack of adult maternal HOXA10 expression during cyclic endometrial development. Reduction of HOXA10 expression in mice using HOXA10 antisense results in diminished implantation proportional to the level of HOXA10 expression, indicating that altered levels of this protein regulates the degree of endometrial receptivity (35).
In the midluteal phase at the time of implantation, HOXA10 mRNA expression is up-regulated in both endometrial glandular and stromal cells in humans (30,38). HOXA10 has diverse effects on several aspects of adult endometrial development such as stromal decidualization, leukocyte infiltration, and pinopod development (34,39). Furthermore, HOXA10 regulates downstream target genes that are also involved in implantation such as β3 integrin, EMX2, and IGFBP-1 (40–42). Defective endometrial HOXA10 expression has been described in association with endometriosis, polycystic ovary syndrome (PCOS), and hydrosalpinges, conditions associated with abnormal implantation (32,43,44). Thus the present study aimed to determine if GnRH antagonists affect HOXA10 expression, a well-characterized marker of endometrial receptivity and one of only a few genes known to be essential for implantation.
Materials and Methods
The study included 19 subjects: 12 subjects undergoing controlled ovulation stimulation with rFSH and a GnRH analogue, and 7 natural cycle controls. The study was approved by the Yale University School of Medicine Human Investigation Committee. Treated subjects were involved in an oocyte donation program. All subjects participated on a voluntary basis. Average age for the oocyte donors was 27 years (range 24–32 years), and average age for the control subjects was 36 years (range 28 – 43 years). Subjects used no medications except for rFSH, GnRH antagonist, GnRH agonist, and human chorionic gonadotropin (hCG), and had no history of endometrial or other uterine disease.
All study subjects underwent ovarian stimulation with rFSH, and dosage was individualized as clinically indicated. Six subjects were treated with the GnRH agonist leuprolide acetate 0.5 mg daily starting on day 21 of the luteal phase, and once suppressed, COH commenced. The GnRH antagonist group started rFSH on cycle day 3, and once the lead follicle reached 13 mm diameter, 0.25 mg of GnRH antagonist was administered daily until hCG administration. For both COH groups, hCG was administered when the lead follicle reached 18 mm diameter. Pipelle endometrial biopsies were performed 11 days after hCG administration. Controls consisted of women in natural cycles, and endometrial biopsies were performed between cycle days 21 to 25 based on LH surge monitoring. Endometrial dating was confirmed based on the criteria of Noyes et al. (45). All endometrial biopsy samples were evaluated with immunohistochemistry (6 GnRH antagonist, 6 GnRH agonist, and 7 control samples) to assess HOXA10 protein expression.
Endometrial tissue was fixed in formalin, embedded in paraffin, cut into 5-μm sections, and mounted onto slides. Slides were deparaffinized and dehydrated through a series of xylene and ethanol washes, followed by permeabilization in 95% cold ethanol. After a 5 minute rinse in distilled water, an antigen-presenting step was performed by steaming the slides in 0.01M sodium citrate buffer for 20 minutes, followed by cooling for 20 minutes. Slides were rinsed for 5 minutes in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 minutes followed by a 5 minute PBST wash. Nonspecific binding was blocked with 1.5% normal horse serum in PBST for 1 hour at room temperature. Slides were incubated in the primary antibody overnight at 4°C. HOXA10 antibody (sc-17159) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Normal goat IgG (Santa Cruz Biotechnology) was used as a negative control for HOXA10 antibody.
Biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). Horse α-goat secondary antibody was applied for 1 hour at 4°C. Slides were washed in PBST, incubated in ABC Elite (Vector Laboratories) for 15 minutes at room temperature, washed in PBST, and incubated for 5 minutes in diaminobenzidine (Vector Laboratories). A 20 second exposure to hematoxylin was used as a counterstain. Slides were rehydrated through 3 minute ethanol and xylene washes and mounted with Permount. Slides from the GnRH antagonist and GnRH agonist treated cycles were processed simultaneously.
The HOXA10 immunohistochemistry results were quantified by three evaluators blinded to treatment regimen. An H-SCORE was determined separately for the glandular cells and stromal cells from 4 high-powered fields on each slide. The H-SCORE was calculated with the following equation: H-SCORE = Σ Pi (i + 1). Intensity (i) of HOXA10 nuclear staining is indicated by a value of 1, 2, or 3 (weak, moderate, or strong respectively), and Pi is the percentage of stained nuclei for each intensity, ranging from 0 – 100% (46,47). The three H-SCORE results for the glands from each slide were averaged, and the three H-SCORE results for the stroma were averaged. The GnRH antagonist, GnRH agonist, and control group glandular and stromal cell H-SCORES were compared using the Kruskal-Wallis One Way Analysis of Variance on Ranks test with Dunn’s multiple-comparison procedure.
Results
The effect of controlled ovarian hyperstimulation with concomitant GnRH antagonist use on endometrial HOXA10 expression, a marker endometrial receptivity, was investigated. Subjects consisted of 12 oocyte donors who underwent COH with concomitant use of a GnRH antagonist (n = 6) or GnRH agonist (n = 6), and 7 natural cycle controls. Peak serum estradiol levels were not significantly different between the 11 GnRH agonist and GnRH antagonist groups (2300 mIU/mL versus 2850 mIU/mL, p = 0.47). Similarly, progesterone levels did not differ between the two groups. Serum LH levels were significantly higher in the GnRH agonist group (1.6 mIU/mL) compared to the GnRH antagonist group (0.3 mIU/mL, p < 0.01).
Immunohistochemistry was employed to evaluate HOXA10 expression in the endometrial glands and stroma cell compartments. Representative immunohistochemistry results are shown in Figure I. HOXA10 expression in the endometrial glands and stroma was quantified, and H-SCORES were determined (Table I). There were no significant differences in HOXA10 expression in the glandular cells between any of the three groups (p = 0.213). However, a significant difference in HOXA10 expression was identified in stromal cells between the three groups (p = 0.003); there was significantly less HOXA10 expression in the stromal cells of subjects treated with GnRH antagonist (mean H-SCORE = 1.50 ± 0.18) compared with the stromal cells in the GnRH agonist group (mean H-SCORE = 2.51 ± 0.12) and with the untreated group (mean H-SCORE = 2.31 ± 0.07) (p < 0.05). Thus immunohistochemistry identified a significant decrease in HOXA10 protein expression in the endometrial stromal cells of women treated with GnRH antagonists.
Figure I. Endometrial HOXA10 Immunohistochemistry.
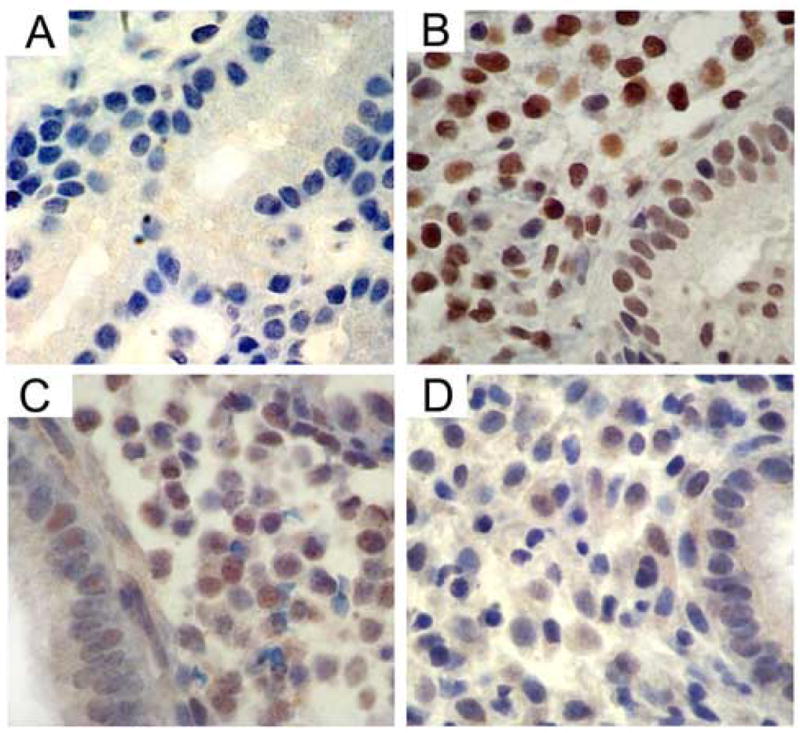
Immunohistochemistry identified HOXA10 protein expression in endometrial glands and stroma. H-SCOREs were determined separately for the glands and stroma. Shown are representative photomicrographs demonstrating HOXA10 expression in endometrium: (A) HOXA10 negative control omitting primary antibody, (B) Natural cycle control, (C) Subject treated with GnRH antagonist, (D) Subject treated with GnRH agonist.
Table 1.
HOXA10 immunohistochemistry mean H-SCOREs ± standard error.
| Antagonist | Agonist | Control | |
|---|---|---|---|
| Glands | 0.59 ± 0.10 | 0.67 ± 0.19 | 0.84 ± 0.09 |
| Stroma | 1.50 ± 0.18a | 2.51 ± 0.12 | 2.31 ± 0.07 |
P < 0.05 for HOXA10 protein expression in GnRH antagonist treated cycles compared with GnRH agonist treated cycles or with controls.
Discussion
Controlled ovarian hyperstimulation is an effective means to produce multiple oocytes, and subsequently multiple high quality embryos, however the effect of the involved medications on endometrial receptivity remains controversial (3,7,14,26). GnRH agonists, and more recently GnRH antagonists, are often used during COH to prevent premature ovulation.
Women undergoing COH cycles using GnRH antagonists produce fewer follicles, thus fewer oocytes and embryos, and when a standardized number of embryos are transferred, lower implantation and pregnancy rates have been observed (25). One meta-analysis of 5 prospective randomized controlled trials (2 using cetrorelix and 3 using ganirelix) compared a fixed GnRH antagonist protocol to the long luteal GnRH agonist protocol, and identified significantly fewer clinical pregnancies per cycle with the GnRH antagonist protocol (OR 0.77, 95% CI 0.61, 0.96) (22). While none of the individual studies showed a significant difference in clinical pregnancy rates between the two protocols, each trial revealed a consistently lower pregnancy rate with the GnRH antagonist protocol. Similarly, Ludwig et al. performed a meta-analysis to evaluate pregnancy rates with GnRH antagonist compared with GnRH agonist and identified an overall lower pregnancy rate per cycle with the antagonist protocol (OR 0.82, 95% CI 0.68, 0.99) (27).
The effect of GnRH antagonists on implantation appears to be dose-responsive. High doses of ganirelix (0.5, 1, 2 mg) have been connected with low implantation rates (17,25). Implantation rates were highest in the 0.25 mg group (21.9%), and lowest in the 2 mg group (1.5%), and the higher doses were associated with higher miscarriage rates (17). In contrast, when embryos were cryopreserved after an ovulation stimulation cycle in which high-dose GnRH antagonists were used and later thawed and transferred, the implantation and pregnancy rates were unaffected by the use of GnRH antagonist during the initial stimulation cycle (20,25). These data suggest an effect of GnRH antagonists on the endometrium and hence, an effect on endometrial receptivity.
Little is known about the effect of GnRH antagonists on the endometrium, therefore we investigated the effect of GnRH antagonist use on endometrial receptivity by evaluating HOXA10 protein expression in endometrial glands and stroma. Endometrial stromal cell HOXA10 protein expression was significantly decreased in cycles using GnRH antagonist compared with cycles using GnRH agonist or natural cycle controls. No difference was noted in glandular cell HOXA10 protein expression among the three groups. Our results identify a potential differential effect of GnRH antagonists on individual endometrial cellular components by demonstrating a negative impact on stromal cells, and no similar effect on glandular cells. This finding may explain the trend towards a lower rate of clinical pregnancies in cycles using GnRH antagonists compared with cycles using GnRH agonists.
In contrast to our results, one recent study demonstrated that endometrial development in oocyte donors treated with a GnRH antagonist was more similar to endometrium from natural cycles than the endometrium from GnRH agonist cycles (16). These investigators used a gene microarray to detect global changes in gene expression, however they reported 2-fold or greater differences in gene expression and would not have detected a small but significant difference in key regulatory genes such as HOXA10 (C. Simon and J. Horcajadas, personal communication).
GnRH antagonists may have a direct or indirect negative effect on the endometrium that could affect implantation (21,22,26). Currently there is no evidence that GnRH antagonists negatively impact oocyte quality, fertilization rates, or embryo quality (21,24,28). Recombinant FSH, GnRH agonist, and GnRH antagonist do not directly alter HOXA10 mRNA expression in vitro; this implies that the effect of GnRH antagonists is not directly on the endometrium, but may be due to altered hormone levels (36).
Despite the impact on endometrial receptivity, there are significant advantages to using GnRH antagonists instead of GnRH agonists. GnRH agonists are initiated in the luteal phase and cause an initial stimulatory effect before acting to down-regulate the GnRH receptors prior to gonadotropin stimulation, and are associated with hypoestrogenic side effects and greater gonadotropin stimulation requirements (23). In comparison, GnRH antagonists act by competitive blockade of pituitary GnRH receptors and cause rapid suppression of FSH and LH. The advantages of GnRH antagonists include a rapid dose-dependent effect, the lack of an initial stimulatory effect, shorter duration of treatment with fewer symptoms of estrogen deprivation, a decrease in gonadotropin requirement for stimulation, and lower risk of developing severe ovarian hyperstimulation syndrome (18,19,21–24,27,28).
The use of GnRH antagonists impaired HOXA10 expression in endometrial stromal cells, and thus may affect endometrial receptivity. However, GnRH antagonists have been shown to be effective, safe, and the therapeutic benefits may outweigh any negative impact, thus these medications should continue to have a role in controlled ovarian stimulation. The impact on endometrial receptivity is likely indirect, suggesting the potential to counter this effect by endocrine means. In conclusion, these results support a molecular basis for the lower pregnancy rates seen clinically with GnRH antagonist use. A greater understanding of GnRH antagonist effects on the endometrium, and further studies investigating other markers of implantation, will help to determine the optimal use of these medications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huisman GJ, Fauser BC, Eijkemans MJ, Pieters MH. Implantation rates after in vitro fertilization and transfer of a maximum of two embryos that have undergone three to five days of culture. Fertil Steril. 2000;73:117–22. doi: 10.1016/s0015-0282(99)00458-6. [DOI] [PubMed] [Google Scholar]
- 2.Schoolcraft WB, Gardner DK. Blastocyst versus day 2 or 3 transfer. Semin Reprod Med. 2001;19:259–68. doi: 10.1055/s-2001-18045. [DOI] [PubMed] [Google Scholar]
- 3.Levi AJ, Drews MR, Bergh PA, Miller BT, Scott RT., Jr Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76:670–4. doi: 10.1016/s0015-0282(01)01988-4. [DOI] [PubMed] [Google Scholar]
- 4.Pattinson HA, Greene CA, Fleetham J, Anderson-Sykes SJ. Exogenous control of the cycle simplifies thawed embryo transfer and results in a pregnancy rate similar to that for natural cycles. Fertil Steril. 1992;58:627–9. doi: 10.1016/s0015-0282(16)55278-9. [DOI] [PubMed] [Google Scholar]
- 5.Pellicer A, Valbuena D, Cano F, Remohi J, Simon C. Lower implantation rates in high responders: evidence for an altered endocrine milieu during the preimplantation period. Fertil Steril. 1996;65:1190–5. doi: 10.1016/s0015-0282(16)58337-x. [DOI] [PubMed] [Google Scholar]
- 6.Queenan JT, Jr, Veeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertil Steril. 1994;62:545–50. doi: 10.1016/s0015-0282(16)56943-x. [DOI] [PubMed] [Google Scholar]
- 7.Simon C, Garcia Velasco JJ, Valbuena D, Peinado JA, Moreno C, Remohi J, et al. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;70:234–9. doi: 10.1016/s0015-0282(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch WJ. Immunolocalization of a gonadotropin-releasing hormone receptor site in murine endometrium that mediates apoptosis. Cell Tissue Res. 1995;282:527–9. doi: 10.1007/BF00318886. [DOI] [PubMed] [Google Scholar]
- 9.Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- 10.Shemesh M. Actions of gonadotrophins on the uterus. Reproduction. 2001;121:835–42. doi: 10.1530/rep.0.1210835. [DOI] [PubMed] [Google Scholar]
- 11.Check JH, Choe JK, Katsoff D, Summers-Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation following in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:416–20. doi: 10.1023/A:1020565408018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–7. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 13.Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. 2003;9:515–22. doi: 10.1093/humupd/dmg045. [DOI] [PubMed] [Google Scholar]
- 14.Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–9. doi: 10.1016/s0015-0282(02)03323-x. [DOI] [PubMed] [Google Scholar]
- 16.Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–3327. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 17.A double-blind, randomized and dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon) The ganirelix dose-finding study group. Hum Reprod. 1998;13:3023–31. [PubMed] [Google Scholar]
- 18.Williams RS, Hillard JB, De Vane G, Yeko T, Kipersztok S, Rhoton-Vlasak A, et al. A randomized, multicenter study comparing the efficacy of recombinant FSH vs recombinant FSH with Ganirelix during superovulation/IUI therapy. Am J Obstet Gynecol. 2004;191:648–51. doi: 10.1016/j.ajog.2004.06.072. discussion 651–3. [DOI] [PubMed] [Google Scholar]
- 19.Fluker M, Grifo J, Leader A, Levy M, Meldrum D, Muasher SJ, et al. Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertil Steril. 2001;75:38–45. doi: 10.1016/s0015-0282(00)01638-1. [DOI] [PubMed] [Google Scholar]
- 20.Seelig AS, Al-Hasani S, Katalinic A, Schopper B, Sturm R, Diedrich K, et al. Comparison of cryopreservation outcome with gonadotropin-releasing hormone agonists or antagonists in the collecting cycle. Fertil Steril. 2002;77:472–5. doi: 10.1016/s0015-0282(01)03008-4. [DOI] [PubMed] [Google Scholar]
- 21.Tarlatzis BC, Bili HN. Gonadotropin-releasing hormone antagonists: impact of IVF practice and potential non-assisted reproductive technology applications. Curr Opin Obstet Gynecol. 2003;15:259–64. doi: 10.1097/00001703-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Al-Inany H, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2001:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Coccia ME, Comparetto C, Bracco GL, Scarselli G. GnRH antagonists. Eur J Obstet Gynecol Reprod Biol. 2004;115 (Suppl 1):S44–56. doi: 10.1016/j.ejogrb.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Diedrich K, Ludwig M, Felberbaum RE. The role of gonadotropin-releasing hormone antagonists in in vitro fertilization. Semin Reprod Med. 2001;19:213–20. doi: 10.1055/s-2001-18040. [DOI] [PubMed] [Google Scholar]
- 25.Gordon K. Gonadotropin-releasing hormone antagonists implications for oocyte quality and uterine receptivity. Ann N Y Acad Sci. 2001;943:49–54. doi: 10.1111/j.1749-6632.2001.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez ER. Embryo implantation and GnRH antagonists: embryo implantation: the Rubicon for GnRH antagonists. Hum Reprod. 2000;15:1211–6. doi: 10.1093/humrep/15.6.1211. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M, Katalinic A, Diedrich K. Use of GnRH antagonists in ovarian stimulation for assisted reproductive technologies compared to the long protocol. Meta-analysis Arch Gynecol Obstet. 2001;265:175–82. doi: 10.1007/s00404-001-0267-2. [DOI] [PubMed] [Google Scholar]
- 28.Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod Update. 2002;8:279–90. doi: 10.1093/humupd/8.3.279. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6:75–9. doi: 10.1093/humupd/6.1.75. [DOI] [PubMed] [Google Scholar]
- 30.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–84. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. Faseb J. 2000;14:1101–8. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 32.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 33.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–45. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 34.Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001;222:538–44. doi: 10.1002/dvdy.1209. [DOI] [PubMed] [Google Scholar]
- 35.Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7:1378–84. doi: 10.1038/sj.gt.3301245. [DOI] [PubMed] [Google Scholar]
- 36.Taylor HS, Daftary GS, Selam B. Endometrial HOXA10 expression after controlled ovarian hyperstimulation with recombinant follicle-stimulating hormone. Fertil Steril. 2003;80 (Suppl 2):839–43. doi: 10.1016/s0015-0282(03)00985-3. [DOI] [PubMed] [Google Scholar]
- 37.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–3. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 38.Sarno JL, Kliman HJ, Taylor HS. HOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab. 2005;90:522–8. doi: 10.1210/jc.2004-0817. [DOI] [PubMed] [Google Scholar]
- 39.Daftary GS, Taylor HS. Pleiotropic effects of Hoxa10 on the functional development of peri-implantation endometrium. Mol Reprod Dev. 2004;67:8–14. doi: 10.1002/mrd.20013. [DOI] [PubMed] [Google Scholar]
- 40.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–9. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 41.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–9. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–43. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- 44.Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril. 2002;78:577–80. doi: 10.1016/s0015-0282(02)03306-x. [DOI] [PubMed] [Google Scholar]
- 45.Noyes RW, Hertig AF, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 46.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 47.Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de-novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod. 2000;15:2180–5. doi: 10.1093/humrep/15.10.2180. [DOI] [PubMed] [Google Scholar]
- 48.Olivennes F, Diedrich K, Frydman R, Felberbaum RE, Howles CM. Safety and efficacy of a 3 mg dose of the GnRH antagonist cetrorelix in preventing premature LH surges: report of two large multicentre, multinational, phase IIIb clinical experiences. Reprod Biomed Online. 2003;6:432–8. doi: 10.1016/s1472-6483(10)62163-3. [DOI] [PubMed] [Google Scholar]


