Abstract
Dendritic cells (DC) are key players in the initiation and modulation of adaptive immune responses due to their ability to acquire and present antigen and stimulate T cells. For the induction of effector T cell functions, antigen must be presented by activated DC. In this study, we have compared uptake of antigen by mouse DC in the presence of different Toll-like receptor (TLR) agonists, which are potent inducers of DC activation. Here we show that the reduction in uptake of soluble antigen in the presence of the viral double-stranded RNA (dsRNA) analogues polyinosinic–polycytidylic acid and Ampligen is independent of TLR-mediated DC activation. A reduction in antigen uptake by bone marrow-derived and splenic DC was also observed in response to other RNA homopolymers such as polyinosinic and polyguanylic acids, which are known inhibitors of scavenger receptor-mediated endocytosis. Pinocytosis and mannose receptor-mediated uptake of soluble antigen were not affected by any of the tested nucleic acids. The reduction in antigen uptake by dsRNA did not negatively influence the T cell stimulating properties of the DC. In summary, we conclude that the decrease in antigen endocytosis observed in the presence of a variety of TLR agonists is independent of TLR signalling and is caused by competition for specific surface receptors that are involved in the uptake of these TLR agonists and the antigen.
Keywords: antigen uptake, dendritic cells, endocytosis, Toll-like receptors
Introduction
Dendritic cells (DC) are professional antigen-presenting cells characterized by their unique ability to stimulate naive T cells. Because of their key role in linking innate immune activation with the induction of adaptive immune responses, ex vivo and in vivo manipulation of DC is of special interest for the development of novel vaccination strategies. In order to induce effector functions in T cells, antigen has to be presented by appropriately activated DC (1). Therefore, there has been much interest in studying the activation of DC and how this affects their ability to take up, process and present exogenous antigens.
In the steady state, immature DC reside in the periphery where they act as sentinels of the immune system. Upon infection, DC sense invading pathogens by means of a germ line encoded set of pattern recognition receptors (PRR), which have the ability to detect microbial signatures in the form of pathogen-associated molecular patterns (PAMPs). The most extensively characterized family of PRR is the Toll-like receptor (TLR) family, which comprises 10 members in humans and mice (2). The microbial signatures recognized by this limited set of TLR include bacterial and fungal cell wall components as well as viral and bacterial nucleic acids. PAMPs present in the cell walls of pathogens are detected by a set of TLR at the cell surface, such as TLR4, which recognizes LPS, and TLR2, which is triggered by lipotechoic acid and peptidoglycans (3–6). In contrast, the TLR subfamily that detects nucleic acids associated with viral and bacterial infection is located in a specialized endosomal compartment and requires the uptake of TLR ligands and the acidification of the endosomal compartment to induce TLR-mediated immune activation (7, 8). Different classes of viral nucleic acids are detected by different endosomal TLR with TLR3 recognizing double-stranded RNA (dsRNA), TLR7 and TLR8 sensing viral single-stranded RNA (ssRNA) and TLR9 discerning viral and bacterial DNA (9–13). The activation of DC via particular PRR during the course of infection directly influences the fate of the T cells with which they interact (14, 15).
The detection of PAMPs initiates a complex maturation programme in DC, which affects the up-regulation of co-stimulatory molecules, the production of pro-inflammatory cytokines, the expression of chemokine receptors and the migratory behaviour of the cells and also influences uptake, processing and presentation of antigens (16). The increased T cell stimulatory activity of activated DC in comparison to immature DC is largely attributable to the up-regulation of co-stimulatory molecules on DC and the induction of pro-inflammatory cytokines such as IL-6 and IL-12 in response to activation (17). In addition, antigen presentation is increased in activated DC and contributes to more efficient T cell stimulation (18). Immature DC are very active in the uptake of exogenous material but do not efficiently process and present antigens in the absence of activating stimuli (16). The majority of MHC class II molecules are recycled in immature DC and are, therefore, present intracellularly (19). Upon activation via TLR, DC undergo a transient increase in endocytosis but then cease to take up new exogenous material (20). The exogenous antigens taken up during this phase are efficiently processed and trafficked into the antigen-presenting pathway in a TLR-controlled phagosome autonomous manner (21). Subsequently, the recycling of MHC class II molecules stops, leading to increased levels of MHC class II complexes at the cell surface of the activated DC presenting the acquired antigens (22). This process enables DC to present a snapshot of the exogenous antigens present in the infected tissue.
This complex process of DC maturation has to be taken into account in the context of vaccine design to ensure the induction of memory T cells with the capacity to differentiate rapidly into effector T cells upon later antigen encounter. Thus, for successful vaccination, the activation of DC by appropriate adjuvants, such as TLR agonists, in parallel to the efficient delivery of antigen is crucial. Since uptake and processing of exogenous antigens cease upon maturation of DC and since presentation of antigen is regulated by TLR-mediated signalling, antigen and TLR agonists have to act in cis in the same endocytic vesicle (21). A previous study reported a reduction in antigen uptake by DC in the presence of TLR3 and TLR4 agonists, which was shown to affect T cell stimulation; yet, it remained unclear whether the observed phenomenon was mediated via TLR activation (23). We, therefore, studied a variety of TLR agonists with regard to their impact on antigen uptake and explored the mechanism by which endocytosis is reduced by certain TLR stimuli such as polyinosinic–polycytidylic acid (polyI:C).
Materials and methods
Reagents
PolyI:C was from GE Healthcare (Chalfont St Giles, UK), R837 and LPS (from Escherichia coli K12) were from Invivogen (Toulouse, France) and CpG-containing oligonucleotide (ODN) 1668 was purchased from MWG (Edersberg, Germany). Polyinosinic acid (polyI), polyguanylic acid (polyG), polyuridylic acid (polyU) and mannan from Saccharomyces cerevisiae were obtained from Sigma (Gillingham, Dorset, UK). Ampligen was from Bioclones (PTY) Ltd (Sandton, Republic of South Africa). Alexa555-conjugated ovalbumin (OVA–Alexa555) and lucifer yellow (LY) were ordered from Invitrogen (Paisley, UK) and 1′-dioctadecyl-3,3,3′,3′tetramethylindo-carbocyanine perchlorate-labelled acetylated low-density lipoprotein (Dil-Ac-LDL) was obtained from Autogen Bioclear (Calne, UK). The following antibodies (all from BD Biosciences, Oxford, UK) were used in the experiments: anti-CD11c, anti-CD4, anti-CD8 and anti-B220. The antibody against PDCA-1 (clone eBio129c) was from eBioscience (Insight Biotechnology Ltd, Wembley, UK) and the antibody recognizing scavenger receptor class A (SR-A; clone 2F8) was purchased from AbD-Serotec (Kidlington, UK) (24).
Animals and cells
C57BL/6 and BALB/c mice were obtained from Harlan UK (Bicester, UK). TIR domain-containing adaptor-inducing IFN-β- (TRIF) and TLR4-deficient mice were bred in the biological service unit at King's College London (25, 26). TLR3-deficient mice were bred at Cancer Research UK (9).
Bone marrow-derived DC (BMDC) were generated from bone marrow cell suspensions in the presence of recombinant granulocyte macrophage colony-stimulating factor (GM-CSF; produced by the Cancer Research UK protein synthesis service) in complete RPMI medium (RPMI 1640 medium containing 10% FCS, 2 mM glutamine, 100 units ml−1 penicillin, 100 μg ml−1 streptomycin and 50 μM 2-mercaptoethanol) as described (27). GM-CSF was produce by the Cancer Research UK protein synthesis service. BMDC were used for experiments at day 5 of cultures.
Splenic DC were enriched from liberase/DNase-digested (both from Roche, Weleyn Garden City, UK) splenocyte preparations using anti-CD11c MACS beads (Miltenyi, Bergisch Gladbach, Germany), following manufacturer's instructions. The different splenic DC subsets were classified into CD4+, CD8+, double-negative (DN) and plasmacytoid DC (pDC) based on their expression of CD4, CD8, B220 and PDCA-1.
Uptake assays
BMDC (1 × 106) were seeded per well into 24-well plates and were incubated with OVA–Alexa555 (5 μg ml−1), Dil-Ac-LDL (0.5 μg ml−1) or LY (0.1 mg ml−1) in the presence or absence of different TLR ligands. After a 4-h incubation, cells were harvested and stained for flow cytometry. Samples were acquired and analysed on a FACSCalibur or a FACSCanto II (BD Biosciences) and the frequency of OVA-, LY- or Dil-Ac-LDL-positive CD11c+ DC was determined.
For the study of mannose receptor-mediated uptake, DC were incubated with varying doses of mannan (300, 30 and 3 μg ml−1) for 1 h prior to the addition of OVA–Alexa555 and TLR agonists. The uptake assay was performed as described above.
Activation assays
BMDC (2 × 105) were seeded per well in 96-well plates and incubated overnight in the presence of polyI:C and CpG 1668 ODN. Supernatants were collected after 18 h of culture and levels of IL-6 were determined by sandwich ELISA (clones MP5-20F3 and MP5-32C11 from BD Biosciences as capture and detection antibody, respectively).
CTL priming
BMDC were incubated overnight with 50 μg ml−1 polyI:C, 0.1 μg ml−1 CpG 1668 or 1 μg ml−1 R837 in the presence of sterile filtered egg white as a source of OVA. Egg white was prepared as described and the concentration of OVA in BMDC cultures was equivalent to 150 μg ml−1 (28). After overnight incubation, 1 × 106 BMDC per mouse were injected intravenously into C57BL/6 recipients. On day 7 after vaccination, the mice were injected intravenously with a 1:1 mixture of splenocytes that had been pulsed with 200 nM of OVA class I peptide (SIINFEKL) or left unpulsed and labelled with 2.5 and 0.25 μM carboxyl fluorescein succinimidyl ester (CFSE), respectively. One day later, mice were sacrificed and the frequencies of the two CFSE-labelled target cell populations were analysed by flow cytometry on a FACSCanto II (BD Biosciences). Antigen-specific killing was calculated using the following formula: (1 − percentage of CFSEpeptide/percentage of CFSEno peptide) × 100.
Statistical analysis
One-way analysis of variance and Dunnett's or Tukey's post test were performed using GraphPad Prism 4. The symbols represent P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***).
Results
Uptake of soluble antigen by BMDC is reduced in the presence of certain TLR agonists
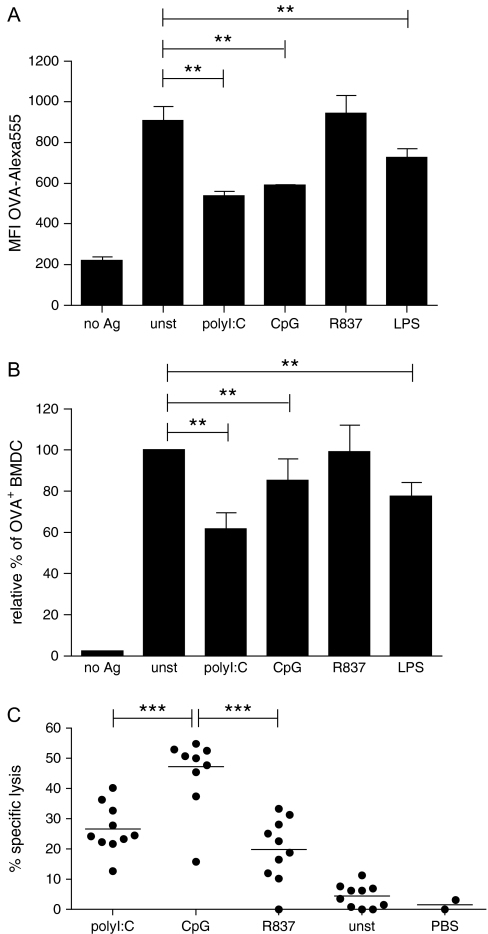
To study the effect of DC activation by different TLR agonists on the uptake of the soluble model antigen OVA, we incubated BMDC with fluorescently labelled OVA in the presence of various TLR agonists at concentrations optimized for maximal DC activation. After 4 h of incubation, cells were harvested and the mean fluorescence intensity for OVA–Alexa555 of CD11c+ cells was determined by flow cytometry to quantify antigen uptake. Activation of BMDC with TLR agonists led to a reduction in antigen uptake in the presence of polyI:C, CpG and LPS (Fig. 1A). Interestingly, the addition of the TLR ligand R837 did not result in reduced OVA endocytosis (Fig. 1A).
Fig. 1.
Reduced uptake of soluble antigen by BMDC in the presence of TLR agonists. Granulocyte macrophage colony-stimulating factor (GM-CSF) BMDC were cultured for 4 h with OVA–Alexa555 (5 μg ml−1) in the presence of polyI:C (50 μg ml−1), CpG (0.5 μg ml−1), R837 (1 μg ml−1), LPS (1 μg ml−1) or left untreated. Cells were stained with anti-CD11c antibody and samples were acquired by flow cytometry. (A) The mean fluorescent intensity of cells for Alexa555 was determined after gating on the CD11c+ cell population. The data of two independent representative experiments were pooled (n = 4) and the standard deviation is indicated. (B) The frequency of OVA–Alexa555+ cells was determined after gating on the CD11c+ cell population. The relative percentage of OVA–Alexa555+ BMDC in samples stimulated with TLR agonists is depicted in relation to the frequency of OVA–Alexa555+ BMDC in the absence of TLR stimulation (unst), which was set to 100%. The results of eight independent experiments were compiled and the standard deviation is indicated. (C) Mice were vaccinated intravenously with GM-CSF BMDC that had been pulsed overnight with OVA in the presence or absence of various TLR ligands. Seven days after vaccination, CFSE-labelled target cells were injected and antigen-specific lysis of peptide-pulsed targets cells versus unpulsed target cells was determined by flow cytometry the following day. The graph compiles data from two independent experiments (n = 10). For statistical analysis, one-way analysis of variance in combination with Dunnett's (A and B) or Tukey's (C) multiple comparison test was used (**P < 0.01, ***P < 0.001).
To compile the results from a set of different experiments, the percentage of CD11c+ BMDC, which were positive for OVA–Alexa555, was determined. The percentage of OVA+ BMDC found in the absence of TLR-mediated activation (unstimulated) was set as 100% and the samples incubated in the presence of TLR agonists were expressed in relation to the unstimulated control sample. This analysis confirmed that co-culture of DC with polyI:C, CpG and LPS reduced the uptake of soluble OVA to 61.8 ± 7.8%, 85.2 ± 10.7% and 77.6 ± 6.9%, respectively, in comparison to unstimulated cells (Fig. 1B). In contrast, no significant reduction in antigen capture in the presence of the TLR7 agonist R837 was observed (Fig. 1B).
To exclude the possibility that a reduction in antigen uptake reflects an increase in cell death in response to the TLR stimuli, we determined the percentage of live cells in the forward versus side scatter dot plots upon analysis of the cells by flow cytometry and also determined the percentage of apoptotic Annexin V+ cells 18 h after stimulation. In both cases, the viability of cells treated with TLR agonists was comparable to that of unstimulated cells (Supplementary Figure 1, available at International Immunology Online). Thus, the reduction in antigen uptake is not due to an increase in cell death.
DC activation in response to the tested concentrations of TLR agonists was confirmed by measuring the induction of IL-6 upon overnight incubation and was seen for all stimuli tested in the assay (data not shown).
Reduction in antigen uptake does not correlate with levels of CTL priming
We were interested in exploring whether the reduction in antigen uptake affects the T cell stimulatory function of DC and thereby negatively influences CTL priming. To address this, we vaccinated mice with BMDC that were pulsed with antigen in the presence and absence of various TLR ligands. One week after vaccination, the induction of a CTL response was determined by in vivo CTL assay. As expected, TLR agonist-treated DC were more potent in inducing CTL than unstimulated control DC irrespective of the TLR agonist that was used for activation (Fig. 1C). However, polyI:C-treated DC were less efficient in inducing CTL responses than CpG-stimulated DC resulting in 26.62 ± 7.99% and 47.21 ± 13.01% of antigen-specific killing of target cells, respectively (Fig. 1C). While the low level of CTL induction for polyI:C-treated BMDC could be caused by reduced antigen presentation due to a decrease in endocytosis in the presence of dsRNA, it also could be a consequence of differences in the activation status of the DC. We determined the induction of IL-6 in response to the different TLR agonists as a quantitative marker for DC activation. While polyI:C-treated DC secreted 4.34 ± 0.14 ng ml−1 IL-6, 56.64 ± 2.98 ng ml−1 IL-6 was detected in the supernatant from the same number of CpG-stimulated DC (data not shown). In comparison, R837 used at the optimal dose of 1 μg ml−1 does not reduce antigen uptake but induces high levels of IL-6 (43.34 ± 0.81 ng ml−1). Nevertheless, vaccination with R837-treated DC only achieves levels of CTL induction similar to polyI:C (Fig. 1C). Thus, there is no correlation between the reduction in antigen uptake, the levels of IL-6 induced and cross-priming of CTL for DC treated with the different TLR agonists. Consequently, we conclude that other, yet unidentified, factors determine the ability of TLR-stimulated DC to induce a CTL response.
Reduction in antigen uptake in the presence of polyI:C is independent of TLR-mediated DC activation and is seen for various viral dsRNA analogues
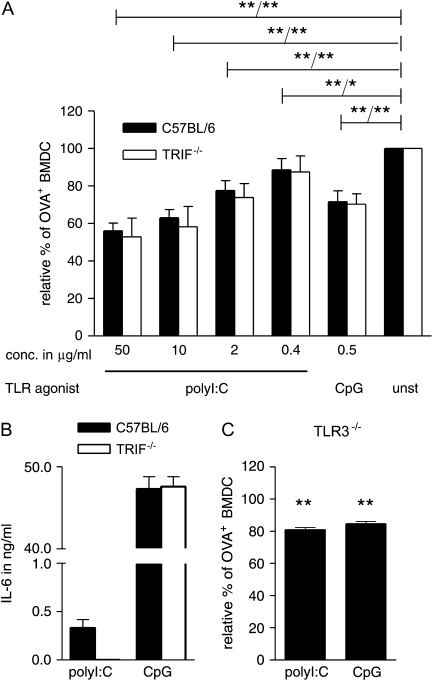
Down-regulation of endocytic activity by DC in response to polyI:C and LPS has been attributed to TRIF-mediated immune activation (23). To test whether the reduction in uptake of soluble antigen in the presence of polyI:C was a consequence of DC activation mediated via TLR3, we compared the reduction in endocytosis of fluorescently labelled OVA in wild-type and TRIF−/− BMDC. TLR3 signals exclusively via the adaptor molecule TRIF and consequently TRIF-deficient cells do not respond to activation with TLR3 agonists (25, 29). Interestingly, we did not find any significant difference in antigen uptake between wild-type and TRIF−/− BMDC (Fig. 2A); however, the induction of IL-6 in response to polyI:C was completely abolished in the TRIF−/− BMDC (Fig. 2B). As expected, TRIF−/− BMDC responded normally to the TLR9 agonist CpG 1668 ODN showing no difference in cytokine induction in response to TLR9-mediated activation (Fig. 2B).
Fig. 2.
The reduction on the uptake of soluble antigen mediated by polyI:C is dose dependent and TRIF independent. (A) GM-CSF BMDC from C57BL/6 and TRIF−/− mice were cultured for 4 h with OVA–Alexa555 (5 μg ml−1) in the presence of the indicated concentrations of polyI:C and CpG. Cells were stained with anti-CD11c antibody and the frequency of OVA–Alexa555+ CD11c+ cells was determined by flow cytometry. The relative percentage of OVA–Alexa555+ BMDC is depicted in relation to BMDC in the absence of TLR agonists. Pooled data from three independent experiments are shown with the standard deviation indicated. The P values are indicated for C57BL/6 and TRIF-deficient BMDC, respectively (wild-type/TRIF). (B) GM-CSF BMDC from C57BL/6 and TRIF−/− mice were cultured overnight in the presence of 50 μg ml−1 polyI:C or 0.5 μg ml−1 CpG. The level of IL-6 in the supernatant of triplicate samples was determined by sandwich ELISA and the standard deviation is indicated. One representative of three independent experiments is shown. (C) GM-CSF BMDC from TLR3−/− mice were treated as described in (A). One representative experiment of three is shown with the standard deviation of triplicate samples indicated. Data were analysed by one-way analysis of variance in combination with Dunnett's multiple comparison test (*P < 0.05, **P < 0.01).
While TLR3 has been described to exclusively signal via TRIF, a role for other TIR domain-containing adaptor molecules cannot be entirely excluded. We, therefore, determined the uptake of fluorescent OVA by TLR3−/− BMDC to confirm our conclusion. As expected, TLR3−/− BMDC showed a reduction in the uptake of OVA in the presence of polyI:C, further strengthening our hypothesis that reduced antigen uptake is independent of TLR-mediated signals (Fig. 2C).
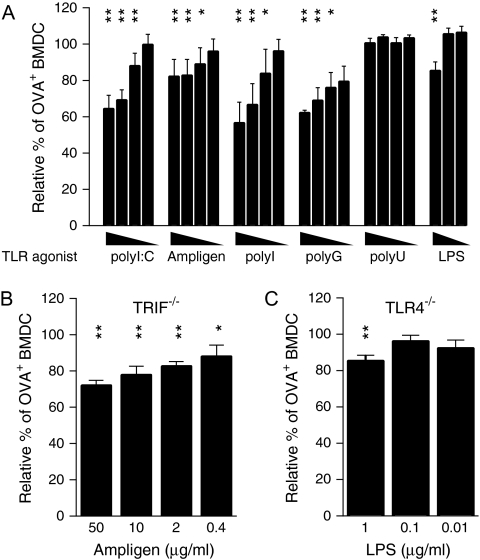
To confirm these data and to exclude activation via cytoplasmic sensors for polyI:C such as retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated gene-5 (MDA5), we tested other agonists for TLR3, namely polyI and Ampligen, a poly(I):poly(C12U) viral dsRNA analogue. The ssRNA homopolymer polyI has recently been described as a TLR3 agonist, while Ampligen has been shown to mediate immune activation exclusively via TLR3 and no other PRR (30, 31). Both alternative stimuli led to a reduction in uptake of soluble OVA (Fig. 3A). While the drop in antigen uptake in response to polyI was similar to the decrease seen for polyI:C, the reduction in the presence of Ampligen was less pronounced.
Fig. 3.
Specific RNA homopolymers partially inhibit antigen uptake by BMDC independently of DC activation. Uptake of OVA–Alexa555 by wild-type (A), TRIF−/− (B) and TLR4−/− (C) BMDC was assessed after 4 h of incubation in the presence of the indicated stimuli. Cells were stained with anti-CD11c antibody and the frequency of OVA–Alexa555+ CD11c+ cells was determined by flow cytometry. The relative percentage of OVA–Alexa555+ BMDC is depicted in relation to BMDC in the absence of TLR agonists. The following concentrations of TLR agonists were used: 0.4, 2, 10 and 50 μg ml−1 for polyI:C, Ampligen, polyI, polyG and polyU and 0.01, 0.1 and 1 μg ml−1 for LPS. The standard deviation is shown and data are representative of at least three independent experiments. Data were analysed by one-way analysis of variance in combination with Dunnett's multiple comparison test (*P < 0.05, **P < 0.01).
Like polyI:C, Ampligen inhibited the uptake of soluble OVA in TRIF-deficient BMDC (Fig. 3B), further supporting the premise that down-regulation of endocytosis takes place independently of DC activation. In accordance with this, inhibition of uptake in the presence of LPS was also observed in TLR4−/− BMDC (Fig. 3C).
Specific RNA homopolymers inhibit uptake of soluble antigen by blocking scavenger receptor-mediated endocytosis
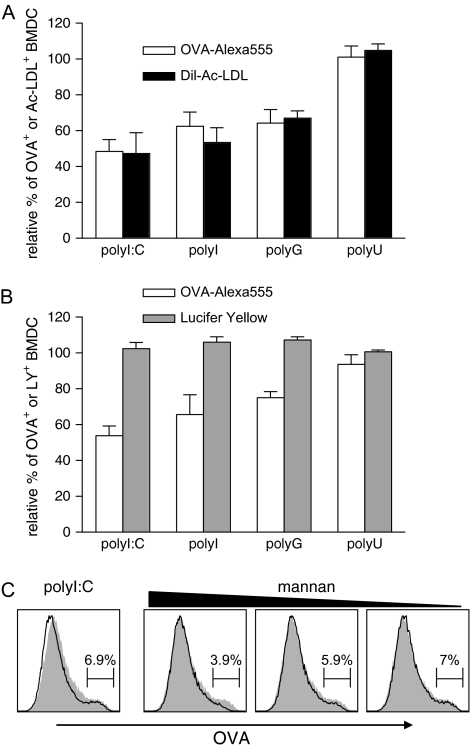
RNA homopolymers with the capacity to form polynucleotide quadruplexes, such as polyI and polyG, have been shown to bind to scavenger receptors and have been used as inhibitors for scavenger receptor-mediated uptake of soluble OVA by macrophages (32, 33). To test whether polyI:C inhibits scavenger receptor-mediated uptake of OVA by DC, we compared the RNA homopolymer-mediated reduction in uptake of soluble OVA with the reduction in uptake of fluorescently labelled acetylated low-density lipoprotein (Dil-Ac-LDL), which is almost exclusively taken up via SR-A by BMDC (34). The level of inhibition of Dil-Ac-LDL uptake was identical to the reduction in OVA endocytosis (Fig. 4A). As expected, the RNA homopolymers polyI:C, polyI and polyG led to similar reductions in OVA–Alexa555 and Dil-Ac-LDL uptake, while polyU, which does not form polynucleotide quadruplexes, did not inhibit uptake of these substances.
Fig. 4.
Scavenger receptor-mediated endocytosis, but not pinocytosis or Mannose receptor-mediated endocytosis, is inhibited by specific RNA homopolymers. (A) GM-CSF BMDC were cultivated for 4 h with OVA–Alexa555 or Dil-Ac-LDL at a final concentration of 5 μg ml−1 in the presence of the indicated RNA homopolymers (50 μg ml−1). Cells were analysed by flow cytometry and the percentage of Alexa555+ or Dil+ CD11c+ BMDC was determined. The results are depicted as relative percentage of OVA+ or Ac-LDL+ BMDC in relation to unstimulated BMDC. The figure compiles results from three independent experiments. (B) Uptake of OVA–Alexa555 (5 μg ml−1) was compared with uptake of LY (0.1 μg ml−1) as described in (A). The figure comprises data from three independent experiments with the standard deviation shown. (C) Mannan at final concentrations of 300, 30 and 3 μg ml−1 was added 1 h prior to the addition of OVA–Alexa555 (5 μg ml−1) to GM-CSF BMDC. As control, cells were incubated with OVA–Alexa555 in the absence of mannan. In addition, some cells were incubated with the antigen in the presence of 50 μg ml−1 polyI:C without mannan. The grey filled histograms depict the uptake of OVA–Alexa555 by CD11c+ BMDC in the absence of polyI:C or mannan. The black histograms show the uptake of OVA in the presence of the indicated reagents. The region depicted in the histograms represents the percentage of CD11c+ cells that have ingested high levels of OVA. The data are representative of four independent experiments.
To confirm that TLR3 ligands such as polyI:C affect scavenger receptor-mediated uptake but do not block other uptake mechanisms, we studied pinocytosis and mannose receptor-mediated uptake in the presence of polyI:C, polyI, polyG and polyU. Uptake of LY, which is taken up exclusively by pinocytosis (35), was determined in the presence and absence of the RNA homopolymers. No reduction in uptake of LY was observed in the presence of any of the tested RNA homopolymers confirming that pinocytosis in BMDC is not affected (Fig. 4B).
Mannose receptor-mediated endocytosis has been shown to be involved in the uptake of OVA by DC (33, 36). To explore the effect of RNA homopolymers on mannose receptor-mediated uptake of OVA, we blocked this pathway by adding increasing doses of mannan to the uptake assay. As expected, this partially blocked the uptake of OVA by BMDC and reduced the frequency of BMDC with high levels of ingested OVA (see histogram regions, Fig. 4C). In contrast, the frequency of BMDC with high levels of endocytosed OVA was not or only slightly affected by the presence of polyI:C, indicating that the TLR3 agonist does not interfere with mannose receptor-mediated uptake of the antigen (Fig. 4C).
Inhibition of scavenger receptor-mediated uptake by RNA homopolymers also occurs in splenic DC
We were interested in examining whether the inhibition of endocytosis by polyI:C also affects antigen uptake by primary splenic DC. Splenic CD11c+ cells were isolated by magnetic enrichment and the expression of the SR-A type I/II (CD204) on the different DC subsets was determined. Immunostaining of the cells with a suitable mix of antibodies allowed for the discrimination of the different splenic DC subsets. All splenic DC subsets expressed CD204 (Fig. 5A). To study the extent of polyI:C-induced reduction in OVA uptake by the different splenic DC subsets, CD11c-enriched splenocytes were cultured in the presence of fluorescently labelled OVA for 4 h before uptake of soluble antigen was analysed by flow cytometry. Interestingly, CD8α+ DC took up far more OVA than the other DC populations while pDC ingested only very little soluble antigen in the same time period (Fig. 5B). In the presence of polyI:C, OVA uptake by CD4+, CD8α+ and DN DC was reduced (Fig. 5B).
Fig. 5.
PolyI:C leads to a reduction in uptake of soluble antigen by splenic DC. CD11c+ splenocytes were isolated by magnetic cell sorting. (A) The different DC subsets were identified by their differential expression of CD4, CD8, B220 and PDCA-1 as depicted. Surface expression of SR-A type I/II (CD204) in the different splenic DC subsets was analysed by flow cytometry. Grey filled and black histograms correspond to immunostaining with an isotype control and anti-CD204 antibody, respectively. (B) Histograms showing the uptake of OVA–Alexa555 by the different splenic DC subsets. Grey filled and black histograms correspond to DC cultured in the absence and presence of OVA–Alexa555, respectively. PolyI:C was used at a final concentration of 50 μg ml−1. (C) Relative percentage of OVA–Alexa555+ CD8α+ DC in relation to CD8α+ DC incubated with the antigen in the absence of TLR agonists. PolyI:C, polyI, polyG and polyU were used at 50 μg ml−1 while CpG was added to final concentration of 0.5 μg ml−1. The figure compiles data from five independent experiments with the standard deviation depicted. For statistical analysis, Dunnett's multiple comparison test was used with the unstimulated cells serving as control group (*P < 0.05, **P < 0.01).
Since the CD8α+ DC subset was the population with the highest rate of OVA uptake, we compared the levels of ingested OVA in the presence and absence of a wide range of TLR ligands and RNA homopolymers for this DC subset. As seen for BMDC, polyI:C, polyI and polyG reduced OVA uptake to 48, 63 and 37% of the levels seen in unstimulated CD8α+ DC, respectively (Fig. 5C). The TLR7 agonist R837 did not affect OVA uptake, while CpG 1668 ODN led to some reduction in antigen endocytosis (80% of OVA+ cells compared with unstimulated DC) similar to that which had been observed for BMDC (Figs 1B and 2A). These results indicate that the TLR3 ligand polyI:C and other RNA homopolymers with the ability to form quadruplexes affect scavenger receptor-mediated uptake of soluble antigens, such as OVA, on primary splenic DC.
Discussion
Here we show that the partial reduction in endocytosis of soluble antigen by DC in the presence of various TLR ligands is independent of DC activation. It has been shown that the transient initial increase in dextran endocytosis is completely or partially dependent on MyD88-mediated DC activation in response to CpG ODN and LPS, respectively (20). In line with these findings, the reduction in endocytosis of cellular material by DC in the presence of polyI:C and LPS has been attributed to TLR- or, more specifically, TRIF-mediated DC activation, since it was absent in response to TLR stimuli signalling exclusively via the adaptor molecule MyD88 (23). We have tested this hypothesis and report here that reduction in uptake of soluble protein in the presence of dsRNA is independent of TRIF-mediated DC activation. While residual activation of DC in response to polyI:C mediated via cytoplasmic PRR such as RIG-I and MDA5 cannot be formally excluded, activation of DC induced by Ampligen is restricted to TLR3 and is, therefore, completely TRIF dependent. Since reduction in endocytic activity in the presence of Ampligen is not affected in TRIF-deficient DC, we conclude that DC activation is not involved in the down-regulation of endocytosis by particular nucleic acid ligands in general.
This conclusion is also supported by the fact that treatment of DC with polyG, another RNA homopolymer, which does not induce DC activation, also leads to a dose-dependent inhibition of endocytosis. RNA homopolymers such as polyG and polyI with the ability to form quadruplex structures have been shown to inhibit scavenger receptor-mediated endocytosis (32). Scavenger receptors bind a broad range of polyanionic molecules and reports have implicated them in the binding of CpG ODN, the lipid A component of LPS and also polyI:C (37–39). This study adds Ampligen, as another RNA polymer, to the list of molecules with an affinity for scavenger receptors.
While binding of polyI:C to scavenger receptors has been shown for epithelial cells (39), it has not so far been shown to affect antigen uptake by DC in the context of DC vaccines. Cellular uptake of polyI:C can be mediated by CD14 or clathrin-dependent endocytosis depending on the cell type that is studied (40, 41). Interestingly, polyI:C competes with specific CpG ODN for clathrin-dependent uptake (41). The fact that polyI:C and CpG ODN use the same uptake mechanism supports our data showing that the reduction in antigen endocytosis is not dependent on TLR-mediated signalling but is due to competition of OVA with nucleic acids such as polyI:C and CpG ODN for the same uptake receptors. Since reduction in endocytic activity of DC in response to TLR-mediated activation is a widely accepted concept, the mechanism behind polyI:C-induced down-regulation of antigen uptake has not been investigated in detail. This study shows, for the first time, that reduced antigen uptake in the presence of specific TLR ligands such as polyI:C is not dependent on DC activation.
We also have addressed the question as to whether a reduction in antigen uptake in response to TLR agonists correlates with reduced cross-priming activity of treated DC in vivo. For uptake of viral antigen from infected cells, it was shown that human DC activated by polyI:C are less efficient in priming CD8 T cells than untreated DC or DC activated with a TLR2 agonist (23). However, in our system studying responses to soluble antigen in a mouse model, DC treated with polyI:C achieved a similar level of CTL activity than DC treated with R837. Since R837 does not reduce uptake of antigen and also induces higher levels of cytokines by the treated DC, this suggests that the reduction in antigen uptake does not directly translate into a reduction in cross-priming activity of the affected DC. Other, yet unidentified, factors seem to influence the ability of DC to induce a robust CTL response.
Recently, it was shown that the different mechanisms involved in OVA uptake dictate the intracellular route of the ingested antigen and its subsequent presentation (33). Antigen internalized by mannose receptor-mediated endocytosis is confined to a stable early endosomal compartment which allows for cross-presentation of exogenous antigen on MHC class I, while antigen that enters the cell by pinocytosis or scavenger receptor-mediated endocytosis undergoes lysosomal degradation and is destined for MHC class II presentation. Our study indicates that polyI:C specifically inhibits uptake via scavenger receptor without affecting mannose receptor-mediated endocytosis or pinocytosis. Thus, in the presence of polyI:C, antigen is mainly ingested by mannose receptor-mediated uptake and/or pinocytosis. Since mannose receptor-mediated uptake of OVA is crucial for cross-presentation of the antigen on MHC class I molecules, it is not surprising that the polyI:C-induced block in scavenger receptor-mediated endocytosis of antigen does not exert negative influence on cross-presentation in our system.
Our data show that not all aspects of DC function altered by TLR agonists are dependent on TLR-mediated activation. Synthetic mimics of TLR ligands have been described previously to have side effects not mediated via the TLR they target. R837 has been shown to bind directly to adenosine receptor and, thereby inhibits adenylyl cyclase activity and polyI:C, in contrast to Ampligen, is known to trigger cytoplasmic PRR in addition to endosomal TLR3 (31, 42, 43). The inhibition of scavenger receptor-mediated endocytosis by polyI:C can be added to this list. Despite its down-modulation of antigen uptake, polyI:C is a potent adjuvant in vivo which induces immune effector functions characteristic for viral infections. This suggests that for the induction of adaptive immunity, the absolute quantity of ingested antigen is less important than the route of antigen uptake and the quality of the co-delivered signals, which regulate and ensure efficient antigen processing and presentation.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
Cancer Research UK to S.D., I.T., B.G.
Acknowledgments
We thank Michael Robson and Caetano Reis e Sousa for the generous provision of TLR4- and TLR3-deficient mice, respectively. We also thank the S.D. laboratory for helpful discussions and Leonie Taams, Linda Klavinskis, Alfonso Martin-Fontecha and Amy Lewis for the critical review of the manuscript. Conflict of Interests: The authors state that there is no financial conflict of interests regarding this publication.
Glossary
Abbreviations
- BMDC
bone marrow-derived DC
- CFSE
carboxyl fluorescein succinimidyl ester
- DC
dendritic cells
- Dil-Ac-LDL
1′-dioctadecyl-3,3,3′,3′tetramethylindo-carbocyanine perchlorate-labelled acetylated low-density lipoprotein
- DN
double negative
- dsRNA
double-stranded RNA
- GM-CSF
granulocyte macrophage colony-stimulating factor
- LY
lucifer yellow
- MDA5
melanoma differentiation-associated gene-5
- ODN
oligonucleotide
- OVA
ovalbumin
- PAMP
pathogen-associated molecular patterns
- pDC
plasmacytoid DC
- polyG
polyguanylic acid
- polyI
polyinosinic acid
- polyI:C
polyinosinic–polycytidylic acid
- polyU
polyuridylic acid
- PRR
pattern recognition receptors
- RIG-I
retinoic acid-inducible gene-I
- SR-A
scavenger receptor class A
- ssRNA
single-stranded RNA
- TLR
Toll-like receptors
- TRIF
TIR domain-containing adaptor-inducing IFN-β
References
- 1.Reis e Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6:476. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 2.Uematsu S, Akira S. Toll-like receptors and innate immunity. J. Mol. Med. 2006;84:712. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 4.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 1999;274:17406. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 5.Underhill DM, Ozinsky A, Hajjar AM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999;163:1. [PubMed] [Google Scholar]
- 7.Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J. Immunol. 1998;160:4755. [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 10.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 11.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 2004;16:21. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 16.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 1999;20:561. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 18.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 19.Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 20.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 21.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 22.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 2000;191:927. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 24.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749. [PubMed] [Google Scholar]
- 27.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boes M, Bertho N, Cerny J, Op den Brouw M, Kirchhausen T, Ploegh H. T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner. J. Immunol. 2003;171:4081. doi: 10.4049/jimmunol.171.8.4081. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Sato S, Mori K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 2002;169:6668. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 30.Marshall-Clarke S, Downes JE, Haga IR, et al. Polyinosinic acid is a ligand for toll-like receptor 3. J. Biol. Chem. 2007;282:24759. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- 31.Gowen BB, Wong MH, Jung KH, et al. TLR3 is essential for the induction of protective immunity against Punta Toro Virus infection by the double-stranded RNA (dsRNA), poly(I:C12U), but not Poly(I:C): differential recognition of synthetic dsRNA molecules. J. Immunol. 2007;178:5200. doi: 10.4049/jimmunol.178.8.5200. [DOI] [PubMed] [Google Scholar]
- 32.Pearson AM, Rich A, Krieger M. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet-stabilized four-stranded helices. J. Biol. Chem. 1993;268:3546. [PubMed] [Google Scholar]
- 33.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 34.Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur. J. Immunol. 2006;36:950. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- 35.Swanson JA, Yirinec BD, Silverstein SC. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J. Cell Biol. 1985;100:851. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 2006;176:6770. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 37.Zhu FG, Reich CF, Pisetsky DS. The role of the macrophage scavenger receptor in immune stimulation by bacterial DNA and synthetic oligonucleotides. Immunology. 2001;103:226. doi: 10.1046/j.1365-2567.2001.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 39.Limmon GV, Arredouani M, McCann KL, Corn Minor RA, Kobzik L, Imani F. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. FASEB J. 2008;22:159. doi: 10.1096/fj.07-8348com. [DOI] [PubMed] [Google Scholar]
- 40.Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24:153. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Itoh K, Watanabe A, Funami K, Seya T, Matsumoto M. The clathrin-mediated endocytic pathway participates in dsRNA-induced IFN-beta production. J. Immunol. 2008;181:5522. doi: 10.4049/jimmunol.181.8.5522. [DOI] [PubMed] [Google Scholar]
- 42.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J. Invest. Dermatol. 2006;126:1338. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 43.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.