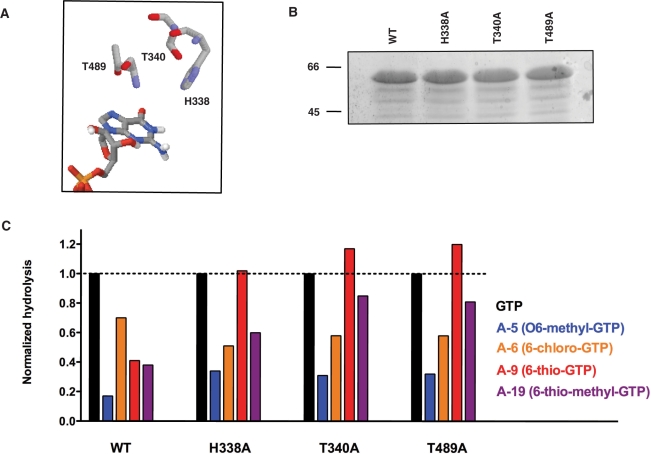
Figure 5.
Steric hindrance caused by analogs harboring large substituents at the 6-oxo position of the guanine ring of GTP. (A) The His338, Thr340 and Thr489 residues of the enzyme are in the vicinity of the 6-oxo group of the GTP substrate. (B) Aliquots (2 µg) of the purified wild-type (WT, lane 1), H338 A (lane 2), Thr340A (lane 3) and Thr489A (lane 4) mutant proteins were analyzed by electrophoresis through a 12.5% polyacrylamide gel containing 0.1% SDS and visualized with Coomassie Blue Dye. The positions and sizes (in kDa) of the size markers are indicated on the left. (C) Normalized phosphohydrolase activities of the WT, H338A, T340A and T489A mutants. The phosphohydrolase-specific activities were calculated from the slopes of the titration curves and normalized to the specific activity for the hydrolysis of GTP by the WT enzyme. GTP, O6-methyl-GTP (A-5), 6-chloro-GTP (A-6), 6-thio-GTP (A-9) and 6-thio-methyl-GTP (A-19) were used as substrates.