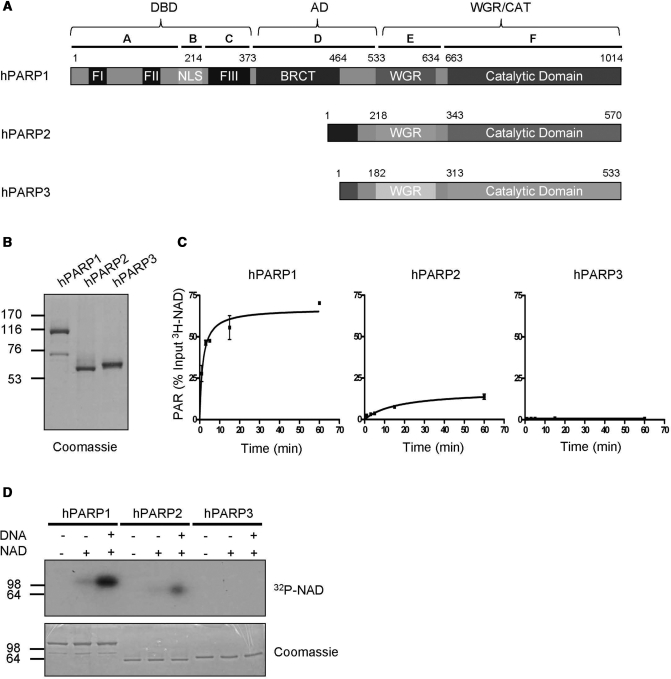
Figure 1.
Purified full-length human PARP1 and PARP2 are enzymatically active. (A) Domain organization of human PARP1, PARP2 and PARP3. Letters A–F indicate domain nomenclature of PARP1 and numbers indicate amino acid positions. (B) Purity of PARP family members after one step affinity chromatography. One microgram of each recombinant, purified protein was used for SDS–PAGE followed by coomassie staining. (C) Time course of PAR formation by different PARP family members. 3H-NAD incorporation into TCA-precipitable polymers was determined by scintillation counts. Substrate concentration: 400 μM 3H-NAD. Reactions were performed in triplicates, error bars represent standard deviations. (D) Auto-modification of different PARP family members detected by autoradiography. Substrate concentration: 100 nM 32P-NAD. Molecular size markers in kilo Daltons are indicated.