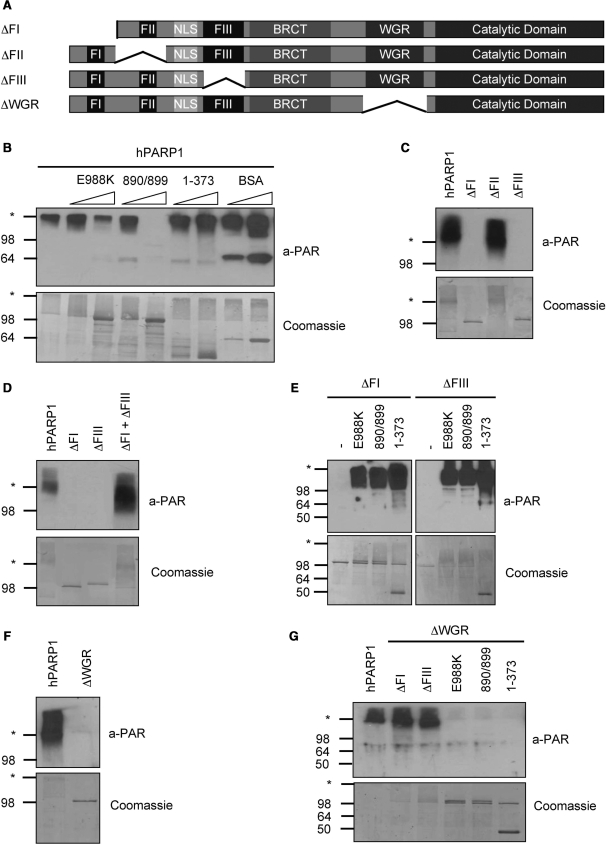
Figure 5.
PARP1 forms a catalytic dimer which requires at least one functional FI and FIII domain for activity. (A) Domain organization of the PARP1 deletion mutants used for this figure. (B) PAR formation by PARP1 when co-incubated with the indicated inactive proteins or fragments at a molar ratio of 1:1 or 1:5. According to the manufacturer, the anti-PAR antibody LP96-10 cross reacts with bovine serum albumin (BSA) (band at around 64 kDa). (C) PAR formation by DBD deletion mutants ΔFI, ΔFII and ΔFIII. (D) PAR formation by a combination of the two DBD deletion mutants ΔFI and ΔFIII. (E) PAR formation by the DBD deletion mutants ΔFI and ΔFIII when they were co-incubated with catalytically inactive PARP1 mutants or with fragment 1–373. (F) PAR formation by PARP1 lacking the WGR domain. (G) PAR formation by PARP1 ΔWGR in combination with DBD deletion mutants, catalytically inactive PARP1 mutants or with fragment 1–373. Molecular size markers in kilo Daltons and the border between stacking and separating gel (asterisk) are indicated.