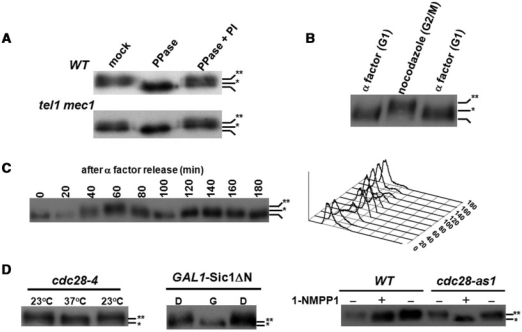
Figure 1.
Cdk1-dependent phosphorylation of Cdc13 in vivo. (A) MEC1/TEL1-independent phosphorylation of Cdc13. Cdc13 was chromosomally tagged with Myc9. Lysates from wild-type and mec1 tel1 strains were immunoprecipitated by a Myc antibody and incubated in PPase buffer, PPase buffer with PPase or PPase buffer containing PPase and PPase inhibitors (PI). Reactions were subjected to a 5.7% SDS–PAGE and transferred for western blot analysis using a Myc antibody. Non-, hypo- and hyper-phosphorylated Cdc13 are marked with none, single and double asterisks, respectively. (B) G2/M phase-specific phosphorylation of Cdc13. Lysates from α factor- or nocodazole-treated cells were extracted by TCA precipitation and Cdc13 phosphorylation was analyzed by western blot analysis. (C) Cell cycle-dependent phosphorylation of Cdc13. Overnight culture was grown to early log phase in YEPD, and arrested in G1 with α factor. When over 90% of the cells, as determined by microscopy, exhibited cell cycle arrest, cells were released into cell cycle. FACS analysis (showed at the right) of this sample indicated that cells had a 1N content of DNA. After release from α factor, cells were collected at 20-min intervals for 180 min and Cdc13 was analyzed by western blot analysis. (D) Cdk1-dependent phosphorylation of Cdc13. Cdc13 phosphorylation was analyzed in cdc28-4, SIC1 overexpressed and cdc28-as1 strains, which all can be manipulated to provide defective Cdk1 activities (37,38,40). cdc28-4 (a temperature sensitive mutant) was synchronized by nocodazole at 23°C and shifted to 37°C for 3 h; pGAL-Sic1 ΔN transformants were treated with dextrose (D) or galactose (G) for 3 h, and W303 (wild-type) and cdc28-as1 were treated with or without 1-NMPP1 for 3 h. Lysates were extracted and Cdc13 phosphorylation was analyzed by western blot analysis.