Abstract
OBJECTIVE
Optimizing glycemic control in diabetic patients undergoing maintenance hemodialysis requires accurate assessment. We hypothesize that 1) 48-h continuous glucose monitoring (CGM) provides additional, clinically relevant, information to that provided by the A1C measurement and 2) glycemic profiles differ significantly between day on and day off dialysis.
RESEARCH DESIGN AND METHODS
With the use of GlucoDay S, 48-h CGM was performed in 19 type 2 diabetic subjects undergoing hemodialysis to capture consecutive 24-h periods on and off dialysis. Energy intake was calculated using food diaries. A1C was assayed by a high-performance liquid chromatography method.
RESULTS
CGM data were available for 17 subjects (13 male) with a mean (range) age of 61.5 years (42–79 years) and diabetes duration of 18.8 years (4–30 years). The 24-h CGM area under the glucose curve and 24-h mean glucose values were significantly higher during the day off dialysis than on dialysis (5,932.1 ± 2,673.6 vs. 4,694 ± 1,988.0 mmol · 3 min−1 · l−1, P = 0.022, and 12.6 ± 5.6 vs. 9.8 ± 3.8 mmol/l, P = 0.013, respectively), independent of energy intake. Asymptomatic hypoglycemia occurred in 4 subjects, 3 within 24 h of dialysis, and the glucose nadir in 14 subjects occurred within 24 h of dialysis.
CONCLUSIONS
Glucose values are significantly lower on dialysis days than on nondialysis days despite similar energy intake. The risk of asymptomatic hypoglycemia was highest within 24 h of dialysis. Physicians caring for patients undergoing hemodialysis need to be aware of this phenomenon and consider enhanced glycemic monitoring after a hemodialysis session. CGM provides glycemic information in addition to A1C, which is potentially relevant to clinical management.
Diabetic nephropathy is the leading cause of end-stage renal failure (ESRF) (1), representing 30–45% of the U.K. and U.S. (2) populations undergoing long-term maintenance hemodialysis. The patients typically are elderly type 2 diabetic patients with established micro- and macrovascular disease (3). Hypoglycemia is common because of impaired renal gluconeogenesis, malnutrition, and the increased half-life of insulin and hypoglycemic agents (4). The annual mortality among diabetic patients undergoing hemodialysis is high and is predominately due to cardiovascular disease (CVD) (2).
Intensive glycemic management delays progression of microvascular disease (5–8) and improves malnutrition (9); however, large randomized controlled trials show no mortality benefit in high-risk groups with CVD (7),(10). Hypoglycemic events increase with intensive treatment and in the presence of CVD can cause fatal dysrhythmia (11). U.K. diabetes guidelines advise against intensive treatment aimed to lower A1C levels <6.5% (12), whereas American guidelines caution against values <7% (13). No evidence-based guidelines for the glycemic targets for diabetic patients with ESRF undergoing long-term maintenance hemodialysis are available.
In patients without ESRF, the A1C value is routinely used to assess long-term glycemic control, and assays are standardized to those used in the Diabetes Control and Complications Trial (14). There is a strong correlation between A1C values and the weighted mean glucose values of the preceding 2–3 months (14).
The validity of the A1C measurement in patients with ESRF undergoing hemodialysis depends on the methodology (15). A number of factors may influence the assay including altered red blood cell (RBC) life span and metabolic and mechanical factors (16). Potential metabolic factors are interference from carbamylated hemoglobin formed in uremia and acetylated hemoglobin formed from long-term aspirin use (17).
A limitation of the A1C value in patients undergoing hemodialysis is that it is not informative regarding glycemic control on the days on and off dialysis. In the U.K., maintenance hemodialysis is typically given in a hospital setting three times a week, with sessions lasting 4–5½ h. The CGM devices that measure glucose every 3 min using a biosensor and a subcutaneous microbore cannula are, in contrast, ideally suited to examine the effect of dialysis on glucose profiles over a 48-h period. Thus, in the present study we test the hypotheses 1) that 48-h CGM provides additional, clinically relevant, information to that provided by the A1C measurement in patients undergoing hemodialysis and 2) that 24-h glucose profiles are different on the day that includes a dialysis session compared with those on a day that does not.
RESEARCH DESIGN AND METHODS
The study was approved by the Hammersmith Hospital Research Ethics Committee, and written informed consent was obtained (Registration No. 2002/6260). Study objectives were to compare glucose profiles from days on and off dialysis using 48-h CGM in type 2 diabetic patients, to examine the association between self-reported food intake and CGM values, and to evaluate glycemic assessment obtained using 48-h CGM in type 2 diabetic patients undergoing maintenance hemodialysis.
Nineteen (14 male) type 2 diabetic subjects were recruited from the maintenance hemodialysis program at Imperial College Kidney and Transplant Institute (ICKTI). Dialysis was performed against a <2 mmol/l glucose-containing dialysate for 4–5½ h during the morning, afternoon, or early evening. Inclusion criteria were a stable hemoglobin level, defined as a <10% change in hemoglobin value and no blood transfusion in the preceding 3 months, a stable dose of erythropoietin, and no hemoglobinopathy. A history of CVD was established as documented ischemic heart disease (history of myocardial infarction, a revascularization procedure, or angiographically proven coronary disease), cerebrovascular disease (history of cerebrovascular accident or transient ischemic attack), or peripheral vascular disease (history of amputation due to gangrene, a revascularization procedure, or peripheral vascular disease proven angiographically or by Doppler ultrasonography).
Blood samples
Blood samples were taken at the start of dialysis for measurement of A1C, hemoglobin, albumin, and urea.
CGM
Day 1.
Subjects attended ICKTI, and before they started dialysis a GlucoDay S CGM device from A.Menarini Diagnostics (Florence, Italy) (18) was fitted and placed in a pouch to be worn around the waist. The CGM biosensor was calibrated retrospectively using capillary blood glucose testing as advised by the manufacturer.
Day 3 (48 h later).
Subjects attended ICKTI, and before their dialysis session the CGM device was removed. The data were downloaded to a computer using dedicated software (GlucoDay S Data Presentation Software).
Exclusion criteria
Exclusion criteria were prospectively defined as type 1 diabetes, intercurrent illness, changes to medication regimen during the monitoring period, or occurrence of prolonged hypoglycemia.
Patient diaries
On day 1, subjects were given a 48-h diary to record the exact time and amount of food, drink, and medications taken during the entire CGM monitoring period, together with any episodes of symptomatic hypoglycemia and all capillary blood glucose results.
Laboratory analysis
A1C measurements were performed in the hospital's clinical biochemistry laboratory using a Diabetes Control and Complications Trial–aligned HA-8160 A1C autoanalyzer (A.Menarini Diagnostics). This analyzer is not subject to interference by urea, as this reverse-phase cation exchange high-performance liquid chromatography (HPLC) method provides good separation of A1C from carbamylated hemoglobin A1. Hemoglobin measurements were performed in the hospital's routine hematology laboratory using a XE2100 autoanalyzer (Sysmex, Kobe, Japan) running a variation of the CyMet hemoglobin absorptiometric method. Serum urea and serum albumin tests were performed on an Architect ci8200 multichannel analyzer (Abbott Diagnostics, North Chicago, IL).
Assessment of glycemic control
The 48-h glucose profiles were quantified, using dedicated software (GlucoDay S Data Presentation Software), as the area under the 3-min glucose curve (AUC) and the mean glucose value. The time periods studied were the first 24-h period starting the first hour of dialysis (day on dialysis) and the 24-h period ending 1 h before the next dialysis session (day off dialysis). The 6-h nocturnal periods from midnight to 6:00 a.m. for each of these 24-h periods were also examined to determine the effect of dialysis. Hypoglycemia, defined as a continuous glucose reading <2.5 mmol/l for >30 min, was identified from the CGM profiles. Subjects were questioned regarding symptoms of hypoglycemia at the end of the CGM period.
Dietetic analysis
Completed food diaries were checked during a dietary consultation with a registered dietitian. Food portions were verified using a pictorial food atlas (19). Comparisons of dietary intake during the 24-h periods on and off dialysis were performed by a data analyst blinded to the study using the Dietplan6 software package (Forestfield Software). The daily energy requirement was calculated to be 30–35 kcal/kg ideal body weight (20).
Statistical analysis
The CGM data were exported into SPSS software (version 14; SPSS for Windows; LEAD Technologies) and tested for normality using the Shapiro-Wilk test.
All normally distributed data are expressed as means ± SD and nonnormally distributed data are expressed as median (range). All comparisons of the glycemic profiles and dietary intake between days on and off dialysis were analyzed using paired Student's t tests. Linear regression analysis was used to assess the relationship between laboratory A1C and weekly erythropoietin dose, serum urea, and serum albumin. The level of significance was defined as P < 0.05.
RESULTS
Nineteen (14 male) subjects were recruited, and 2 were subsequently excluded, one because of repeated hypoglycemia during both monitoring periods and one because of CGM technical failure. The age, duration of diabetes, and years of dialysis [mean ± SD (range)] of the 17 (13 male) subjects included were 61.5 ± 8.8 years (42–79 years), 18.8 ± 7.6 years (4–30 years), and 4 ± 2.6 years (0.5–10.2 years), respectively. Previous CVD history, diabetes medications, erythropoietin dose, A1C, hemoglobin, and urea values are given in Table 1.
Table 1.
Clinical details of 17 subjects whose CGM data were included in the final analysis
| n | Age (years) | Sex | Duration of diabetes (years) | Urea (mmol/l) | CVD (yes/no)* | Medication | Hb (g/dl) | A1C (%) | Time undergoing dialysis (years) | Erythropoietin dose (μg/week) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 20 | 5.1 | Yes | Gliclazide, 40 mg b.i.d. | 13.4 | 5.1 | 1.2 | 30 |
| 2 | 59 | M | 18 | 19.3 | Yes | Gliclazide, 80 mg q.d. | 11.3 | 5.3 | 3.7 | 80 |
| 3 | 65 | M | 6 | 15.1 | Yes | Diet | 12.9 | 6 | 1.3 | 15 |
| 4 | 72 | M | 16 | 22.3 | Yes | Gliclazide, 80 mg q.d. | 9.9 | 6 | 3 | 100 |
| 5 | 63 | M | 20 | 23.0 | Yes | Humulin M3 (8 units b.i.d.) | 14.6 | 6.4 | 3.6 | 30 |
| 6 | 52 | M | 18 | 28.2 | Yes | Gliclazide, 40 mg b.i.d. | 15.1 | 6.5 | 3.7 | 30 |
| 7 | 65 | M | 24 | 14.0 | Yes | NovoRapid (10 units a.m.), Glargine (35 units p.m.) | 12.1 | 6.6 | 2.8 | 60 |
| 8 | 68 | M | 26 | 14.0 | Yes | NovoMix 30 (10 units b.i.d.) | 10.9 | 5.6 | 7.7 | 30 |
| 9 | 65 | M | 30 | 17.9 | Yes | Mixtard 30 (10 units, 8 units) | 12 | 6.7 | 5.4 | 10 |
| 10 | 67 | M | 18 | 24.0 | Yes | Gliclazide, 40 mg b.i.d. | 13.1 | 6.7 | 10.2 | 60 |
| 11 | 58 | F | 9 | 17.0 | No | Mixtard 50 (23 units, 24 units) | 9.3 | 7.4 | 0.5 | 80 |
| 12 | 53 | M | 13 | 26.7 | Yes | Gliclazide, 160 mg b.i.d. | 11.7 | 7.9 | 2.7 | 40 |
| 13 | 79 | M | 22 | 21.9 | Yes | Mixtard 30 (18 units, 12 units) | 14 | 8 | 6.8 | 20 |
| 14 | 42 | M | 26 | 16.0 | Yes | Mixtard 30 (18 units, 12 units) | 13.4 | 8.5 | 3.9 | 15 |
| 15 | 65 | F | 30 | 13.2 | Yes | Mixtard 30 (6 units b.i.d.) | 13.8 | 7.3 | 6.2 | 50 |
| 16 | 65 | F | 4 | 14.5 | Yes | Gliclazide, 160 mg b.i.d. | 12.4 | 8.9 | 3.3 | 30 |
| 17 | 54 | F | 19 | 17.8 | No | NovoMix 30 (16 units, 10 units) | 11.7 | 9.2 | 1.7 | 60 |
*Documented history of vascular disease defined as ischemic heart disease (history of myocardial infarction, revascularization procedure or angiographically proven coronary disease), cerebrovascular disease (history of cerebrovascular accident or transient ischemia attack) or peripheral vascular disease (history of amputation due to gangrene, revascularization procedure, or peripheral vascular disease proven angiographically or by Doppler ultrasonography). F, female; M, male.
A1C values
The A1C (mean ± SD) was 6.9 ± 1.2% (range 5.1–9.2%), with seven subjects having A1C ≤6.5% (Table 1). Results of linear regression analysis among A1C and erythropoietin dose, serum albumin, and urea were not significant (r2 = 0.17, P = 0.0995; r2 = 0.161, P = 0.536; and r2 = 0.163, P = 0.533, respectively).
Hemoglobin values
Mean ± SD hemoglobin was 12.4 ± 1.6 g/l (range 9.3–15.1 g/l).
Analysis of glycemic profiles
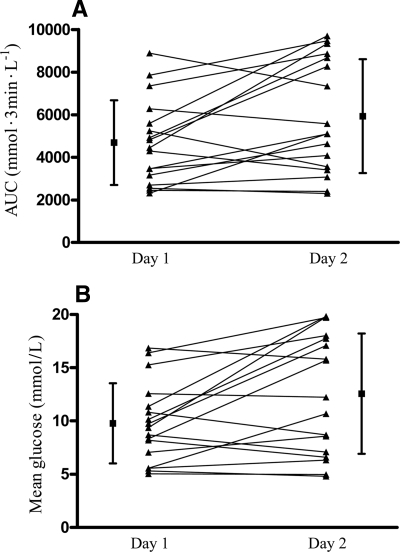
The 24-h AUC glucose values and mean 24-h CGM data were significantly higher the day off dialysis than the day on dialysis (5,932.1 ± 2,673.6 vs. 4,694 ± 1,988 mmol · 3 min−1 · l−1, P = 0.022, and 12.6 ± 5.6 vs. 9.8 ± 3.8 mmol/l, P = 0.013, respectively) (Fig. 1). The difference in the 24-h mean glucose levels for the day off dialysis to the day on dialysis ranged from −2.1 to 10.4 mmol/l.
Figure 1.
CGM data for day on (day 1) and day off (day 2) dialysis, expressed as AUC glucose (A) and mean glucose (B) for each 24-h period. ▲—▲, data for individual subjects; ■, mean ± SD for each 24-h period. A: Mean ± SD area under the 3-min glucose curve for the whole study group was 5,932.1 ± 2,673.6 mmol · 3 min−1 · l−1 on the day off dialysis vs. 4,694 ± 1,988 mmol · 3 min−1 · l−1 on the day on dialysis (P = 0.022). B: Mean ± SD CGM glucose values for the whole group were 12.6 ± 5.6 mmol/l on the day off dialysis vs. 9.8 ± 3.8 mmol/l on the day on dialysis (P = 0.013).
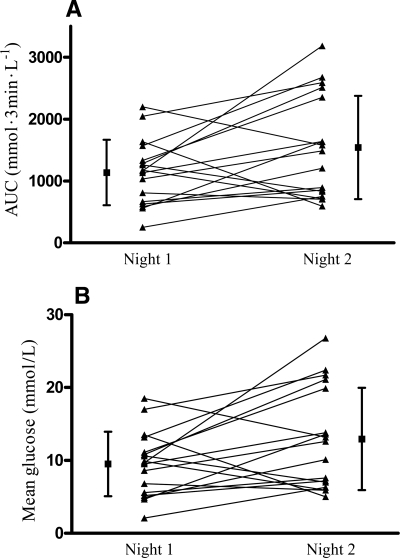
The AUC glucose profiles and the mean glucose values for the 6-h nocturnal period from midnight to 6:00 a.m. were significantly higher for the second than for the first night (1,541 ± 834 vs. 1,137 ± 529 mmol · 3 min−1 · l−1, P < 0.05, and 12.9 ± 7.0 vs. 9.5 ± 4.4 mmol/l, P < 0.05, respectively), with a median 6-h mean nocturnal glucose difference of 4.2 mmol/l (range −8.5 to 17.1 mmol/l) (Fig. 2).
Figure 2.
Nocturnal CGM data for the 6-h period from midnight to 6:00 a.m. for day on (night 1) and day off (night 2) dialysis, expressed as AUC glucose (A) and mean glucose (B). ▲—▲, data for individual subjects; ■, mean ± SD for each 24-h period. A: Mean ± SD area under the 3-min glucose curve for the whole study group was 1,541 ± 834 mmol · 3 min−1 · l−1 for the night of the day off dialysis (night 2) vs. 1,137 ± 529 mmol · 3 min−1 · l−1 for the night of the day on dialysis (night 1) (P < 0.05). B: Mean ± SD CGM glucose values for the whole group were 12.9 ± 7.0 mmol/l on night 2 vs. 9.5 ± 4.4 mmol/l on night 1.
Analysis of hypoglycemia
Four of the 17 subjects had CGM recordings of <2.5 mmol/l for >30 min; in 3 subjects, this recording occurred in the first 24-h monitoring period. Examination of individual CGM profiles showed that 14 of 17 subjects reached their glucose nadir (range 1.38–9.81 mmol/l) within the first 24 h, with 10 of 17 having their lowest reading within 12 h of starting dialysis. No subject reported any episode of symptomatic hypoglycemia.
Analysis of the food diaries
Two subjects failed to complete their 48-h food diaries (subjects 6 and 15). Analysis of the 15 completed diaries showed no significant difference between recorded dietary intakes for the day on dialysis and the day off dialysis (1,636 ± 603 vs. 1,702 ± 559 kcal, respectively, P = 0.596). There was no trend toward greater food intake on either day, with 7 subjects recording a greater calorie intake during the day on dialysis versus 8 during the day off dialysis. The timing of the dialysis shift did not appear to influence the energy intake (data not shown). The total energy intake for each subject was significantly lower, both on dialysis days (mean 1,636 kcal/day) and off dialysis days (mean 1,702 kcal/day), than the estimated mean energy requirement (2,000 kcal/day) (P = 0.01; data not shown).
Medications
No subject recorded a change in frequency or dosing of medications, including insulin, on the 2 days.
CONCLUSIONS
The need to balance glycemic targets to avoid hypoglycemia with the risks of microvascular disease from hyperglycemia requires accurate glycemic assessment. This is especially so for diabetic patients undergoing maintenance hemodialysis, who have a high prevalence of microvascular and macrovascular disease and an increased risk of asymptomatic hypoglycemia during hemodialysis (21). The use of continuous subcutaneous glucose monitors is ideally suited for diabetic patients undergoing hemodialysis because, unlike A1C measurements, they can examine short-term glycemic changes around the time of dialysis and are unaffected by urea, RBC life span, and RBC production.
The need to set the appropriate glycemic targets for type 2 diabetic patients with a high CVD risk was highlighted by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (7) and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) (8) randomized controlled trials. These studies recruited subjects at high risk of CVD, and neither showed any CVD benefit from targeting A1C levels <6.5 and 7%, respectively. The ACCORD study actually showed a small increase in overall mortality when A1C <6.5% was targeted. That low A1C levels may not confer survival benefit in ESRF was also suggested by a 1-year follow-up study of 23,000 American diabetic subjects (22). In contrast, good glycemic control before dialysis does appear to have some CVD benefit (23). Thus, it may be necessary for A1C targets originally based on low CVD risk populations without chronic kidney disease to be reevaluated for diabetic patients undergoing hemodialysis.
The A1C is a measure of the irreversible nonenzymatic glycation product of one or both NH2-terminal valines of the β-hemoglobin chain. In ESRF, the A1C assay can be affected by interference from carbamylated hemoglobin formed from urea-derived isocyanate that accumulates in uremia (24). However, advances in reverse-phase cation exchange HPLC analyzers, as used in this study, allow for greater hemoglobin peak separation (25).
Shortened RBC life span or increased RBC production (16) can occur in ESRF, and both can falsely lower A1C values by reducing the RBC glycemic exposure time. However, a study of 23 patients undergoing hemodialysis who were receiving regular erythropoietin therapy and had stable hemoglobin values concluded that the ambient glucose concentration rather than RBC life span was the major determinant, as no correlation between RBC life span and A1C measured by either immunoassay or HPLC was shown (26). Starting or increasing erythropoietin treatment could, by increasing RBC production, falsely lower A1C values by increasing the proportion of younger RBCs and thereby reducing glucose exposure time. In the present study, all subjects had stable hemoglobin values and their erythropoietin doses had remained constant over the preceding 3 months.
Furthermore, the A1C value may be less informative in the type 2 diabetic population undergoing maintenance hemodialysis and may be less easily translated into mean glycemic values than in other populations. The A1C Derived Average Glucose Study Group (ADAG) investigators recently reported (27) that A1C levels can be converted to average glucose levels in type 2 diabetic patients. However, patients with chronic kidney disease were excluded from this study, and it is possible that the metabolic fluctuations seen with hemodialysis may weaken the relationship between A1C and average glucose. CGM is one alternative to A1C for assessing glycemia.
The present study showed that over a 2-day period the GlucoDay S CGM device recorded significantly higher glucose profiles on the day off dialysis than the day on dialysis. The CGM glucose values during the first 24-h monitoring period (day on dialysis), including the 6-h nocturnal period, were significantly less than those for the second 24-h monitoring period. During the hours of midnight to 6.00 a.m., the only common time all subjects were resting and not eating, the magnitude of this difference ranged from −8.5 to 17.1 mmol/l, with a median difference of 4.2 mmol/l. These differences in glucose profiles were not explained by the difference in 24-h energy intake, changes in medication, or dialysis shift. However, there are potential limitations regarding the accuracy of the food diaries, as data were collected over 48 h only and were self-reported. The food data did highlight the fact that all subjects were likely to be malnourished because they consumed less than their recommended intake.
The CGM data also showed that 4 subjects had hypoglycemia (<2.5 mmol/l over ≥30 min) and that this occurred within 24 h of dialysis in three subjects. The lowest glucose recording for 14 subjects was within the first 24-h period, with the majority being within 12 h of dialysis. Thus, the results of our study suggest that type 2 diabetic patients undergoing maintenance hemodialysis, who already have a very high risk of cardiovascular morbidity and mortality, may have an increased risk of hypoglycemia in the 24-h period after a dialysis session. Current renal practice includes assessment of glycemic control by blood glucose measurements while the patient is undergoing hemodialysis. However, physicians caring for these patients need to be aware of this phenomenon and consider enhanced monitoring for these patients who may develop hypoglycemia several hours after they have left the dialysis unit.
The subjects in this study were typical of the U.K. type 2 diabetic population with a long duration of diabetes (18.8 ± 7.6 years) and high prevalence rates of established vascular disease (15 of 17 subjects) who are undergoing hemodialysis. Our preliminary study suggests that CGM offers clinically useful data for such a high-risk group. Larger studies on populations undergoing hemodialysis will be required to determine whether data from CGM should be used for medication adjustments around dialysis days to optimize glycemic control and avoid hypoglycemia.
In summary, as glycemic targets become redefined to avoid overaggressive management in individuals with high CVD risk, it is important that the measurements of glycemic control in patients undergoing hemodialysis are as informative as possible.
We are grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme. S.K.-A. is funded by the King Faisal Foundation. J.J.O.T. is a British Heart Foundation intermediate fellow. The study was supported by the Biomedical Research Centre at Imperial College's Academic Health Science Centre.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
We thank A.Menarini Diagnostics for providing us with the CGM monitors.
Part of the work was presented in abstract form at the annual meeting of The Renal Association, Glasgow, Scotland, 23–16 May 2008; at the autumn meeting of the Association of British Clinical Diabetologists, London, England, 27–28 November 2008; and at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038– 2047 [DOI] [PubMed] [Google Scholar]
- 2. United States Renal Data System. USRDS 2007 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States [article online], 2007. Available from http://www.usrds.org/adr.htm. Accessed 12 September 2008
- 3. Hakim RM, Levin N: Malnutrition in hemodialysis patients. Am J Kidney Dis 1993; 21: 125– 137 [DOI] [PubMed] [Google Scholar]
- 4. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007; 49: S12– S154 [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2002; 25: S28– S32 [DOI] [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643– 2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560– 2572 [DOI] [PubMed] [Google Scholar]
- 9. Cano NJ, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Fouque D, Laville M, Leverve XM: Malnutrition in hemodialysis diabetic patients: evaluation and prognostic influence. Kidney Int 2002; 62: 593– 601 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. Intense blood glucose control yields no significant effect on CVD reduction in VA Diabetes Trial [article online], 2008. Available from http://www.diabetes.org/for-media/pr-intense-blood-glucose-control-yields-no-significant-effect-on-cvd-reduction.jsp. Accessed 12 Sep- tember 2008
- 11. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 12. NICE, National Institute of Health and Clinical Excellence. Clinical guidelines for the management of type 2 diabetes [article online], 2008. Available from http://www.nice.org.uk/CG66. Accessed on 12 September 2008
- 13. American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007; 30: S4– S41 [DOI] [PubMed] [Google Scholar]
- 14. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE: Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002; 25: 275– 278 [DOI] [PubMed] [Google Scholar]
- 15. Little RR, Tennill AL, Rohlfing C, Wiedmyere HM, Khanna R, Goel S, Agrawal A, Madsen R, Goldstein DE: Can glycohemoglobin be used to assess glycemic control in patients with chronic renal failure? Clin Chem 2002; 48: 784– 786 [PubMed] [Google Scholar]
- 16. Nissenson AR, Fine RN: Dialysis Therapy. 3rd ed. Philadelphia, Hanley & Belfus, 2002 [Google Scholar]
- 17. Bry L, Chen PC, Sacks DB: Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001; 47: 153– 163 [PubMed] [Google Scholar]
- 18. Maran A, Crepaldi C, Tiengo A, Grassi G, Vitali E, Pagano G, Bistoni S, Calabrese G, Santeusanio F, Leonetti F, Ribaudo M, DiMario U, Annuzzi G, Genovese S, Ricardi G, Previti M, Cucinotta D, Giorgino F, Bellomo A, Giorgino R, Poscia A, Varalli M: Continuous subcutaneous glucose monitoring in diabetic patients: a multicenter analysis. Diabetes Care 2002; 25: 347– 352 [DOI] [PubMed] [Google Scholar]
- 19. Nelson M, Atkinson M, Meyer J. Nutritional Epidemiology Group UK. Food Portion Sizes: A Photographic Atlas. London, MAFF Publications, 1997 [Google Scholar]
- 20. Renal Association. Treatment of Adults and Children with Renal Failure: Standards and Audit Measures. 3rd ed. London, Royal College of Physicians of London and the Renal Association, 2002 [Google Scholar]
- 21. Takahashi A, Kubota T, Shibahara N, Terasaki J, Kagitani M, Ueda H, Inoue T, Katsuoka Y: The mechanism of hypoglycemia caused by hemodialysis. Clin Nephrol 2004; 62: 362– 368 [DOI] [PubMed] [Google Scholar]
- 22. Williams ME, Lacson E, Jr, Teng M, Ofsthun N, Lazarus JM: Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006; 70: 1503– 1509 [DOI] [PubMed] [Google Scholar]
- 23. Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, Shoji T, Nishizawa Y: Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care 2006; 29: 1496– 1500 [DOI] [PubMed] [Google Scholar]
- 24. Lee KF, Szeto YT, Benzie IF: Glycohaemoglobin measurement: methodological differences in relation to interference by urea. Acta Diabetol 2002; 39: 35– 39 [DOI] [PubMed] [Google Scholar]
- 25. Schnedl WJ, Lahousen T, Wallner SJ, Krause R, Lipp RW: Silent hemoglobin variants and determination of HbA1c with the high-resolution program of the HPLC HA-8160 hemoglobin analyzer. Clin Biochem 2005; 38: 88– 91 [DOI] [PubMed] [Google Scholar]
- 26. Joy MS, Cefalu WT, Hogan SL, Nachman PH: Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39: 297– 307 [DOI] [PubMed] [Google Scholar]
- 27. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group: Translating the A1c assay into estimated average glucose values. Diabetes Care 2008; 31: 1473– 1478 [DOI] [PMC free article] [PubMed] [Google Scholar]