Abstract
Study objectives:
To explore the acute systemic inflammatory and anabolic effects of cycling in hospital admitted patients with chronic obstructive pulmonary disease (COPD) and in patients with clinically stable disease.
Design:
Cross-sectional comparative study.
Setting:
University Hospital Gasthuisberg, a tertiary care setting.
Patients:
16 patients with clinically stable COPD (no acute exacerbation in the past 12 weeks; median age: 73 years (IQR: 60 to 75); median forced expiratory volume in the first second (FEV1): 45% predicted (IQR: 33 to 58)) and 14 patients who were admitted to a hospital due an acute exacerbation of COPD (median age: 65 years (IQR: 59 to 74); median FEV1 to on day 8 of hospital stay: 41% predicted (IQR: 33 to 54)).
Interventions:
None.
Measurements and results:
Circulating levels of C reactive protein, interleukin 6, interleukin 8 and insulin-like growth factor I were determined before, at the end and 2 and 30 minutes after a symptom-limited peak cycling test and before, at the end and 2 and 30 minutes after a symptom-limited constant-work-rate cycling test at 70% of the peak load. Non-significant changes in the circulating markers of inflammation and anabolism were found during or up to 30 minutes after ceasing the peak or constant-work-rate cycling exercise tests. The systemic responses of the hospitalized patients with COPD did not differ from those with clinically stable disease.
Conclusions:
High-intensity cycling exercises did not increase the circulating levels of inflammatory markers in patients with chronic obstructive pulmonary disease, irrespective of their clinical stability.
Keywords: C reactive protein, interleukin 6, interleukin 8, insulin-like growth factor 1
Introduction
Patients with chronic obstructive pulmonary disease (COPD) have shown to have a systemic low-grade inflammation in stable condition (Gan et al 2004), which transiently increases during acute exacerbations of COPD (Wedzicha et al 2000; Creutzberg et al 2000; Dentener et al 2001; Spruit et al 2003). Low-grade systemic inflammation may also be further aggravated during or following high-intensity exercise bouts. In fact, increased systemic inflammation has been observed in patients with clinically stable COPD following 11 minutes of cycling at only ∼40% of peak external work rate as compared to baseline levels (Rabinovich et al 2003). The acute systemic effects of a single exercise bout may be more apparent in hospitalized patients with COPD, but has never been studied. Nevertheless, an increased systemic inflammation following intensive exercises may also be expected in these patients and may result in additional increased plasma levels of fibrinogen (Wedzicha et al 2000), energy disbalance (Creutzberg et al 2000) and skeletal muscle weakness (Spruit et al 2003). Moreover, it may neutralize the beneficial effects that exercise training may have in these patients (Man et al 2004). Finally, exercise-induced cytokines may directly inhibit the activity of one of the major growth promoting factors (eg, insulin-like growth factor I (IGF-I)) (Fernandez-Celemin et al 2002). Sequentially, this may reduce its circulating levels, as previously found in healthy adolescent boys (Nemet et al 2002) and in patients with cystic fibrosis (Tirakitsoontorn et al 2001). To date, the acute effects of cycling at a high intensity on circulating levels of IGF-I are unknown in patients with clinically stable COPD or during an acute exacerbation.
Given the possible role of increased systemic inflammation and reduced systemic markers of the somatotrophic axis in the development and/or maintenance of skeletal muscle weakness in patients with COPD (Eid et al 2001; Debigare et al 2001, 2003; Spruit et al 2003), the present study was undertaken to further explore the acute systemic effects of cycling in hospital admitted patients with COPD and in those with stable disease. The authors hypothesized that COPD patients who are admitted to the hospital due to an acute exacerbation would have a higher inflammatory response to a single exercise bout as compared to patients with clinically stable COPD.
Materials and methods
Patients
Thirty patients with moderate-to-severe COPD (forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 70%) gave informed consent to participate in the present study, which was approved by the Medical Ethical Committee of the University Hospitals Leuven. Fourteen patients (10 men) were hospitalized while the remaining 16 patients (13 men) did not have an acute COPD exacerbation during at least 12 weeks preceding the tests.
Patients with COPD attended the emergency room due to a sustained worsening of the their condition, from the stable state and beyond normal day-to-day variations, that was acute in onset and required a change in regular medication (Rodriguez-Roisin 2000). The decision to admit patients to the hospital was made by the attending chest physician who was not familiar with the present study protocol. In 2 patients the presence of purulent sputum was documented in the patients’ file. In addition, 2 patients were classified as having a type 1 exacerbation, 3 patients had a type 2 exacerbation, while 9 others had a type 3 exacerbation (Anthonisen et al 1987). Median circulating levels of C-reactive protein was 62.2 mg/l at the emergency room. Hospital admitted patients received 32-milligram · day−1 oral methylprednisolone for 1 week, followed by 24-milligram · day−1 for a period of 4 days and a subsequent decrease of 4-milligram · week−1. No physical exercises were given during the hospital stay.
The acute systemic effects of exercise can be influenced by the intensity and type of the exercise bout (Schwarz et al 1996; Ostrowski et al 2000). Therefore, the patients were asked to perform two different exercise tests on two separate days: a symptom-limited peak exercise test (at day 8 of hospitalization) and a symptom-limited constant-work-rate exercise test at ~70% of the peak external work rate (2–3 days later). In addition, pulmonary function, quadriceps peak torque and the distance walked in 6 minutes were assessed as described previously (Gosselink et al 1996). All procedures were in accordance with the recommendations found in the World Medical Association declaration of Helsinki (Anon 1997).
Symptom-limited peak exercise test
Peak exercise capacity was assessed by a symptom-limited peak exercise test on an electronically braked cycle-ergometer (Ergoline D-72475, Bitz, Germany). After a 2-minute resting period and 3 minutes of unloaded cycling, participants started at 20 watts and cycled until symptom limitation at an incremental workload (+10 watts/minute). Oxygen uptake (Vmax 29C Viasys Meda N.V., Edegem, Belgium), heart rate (Mac VS/ST, Marquette Electronics Inc.) and transcutaneous oxygen saturation (Datex-Ohmeda 3900) were measured during the test. At the end of the test Borg symptom scores for dyspnoea and fatigue were obtained from all participants (Borg 1998). Peak external work rate and peak oxygen uptake were normalized for height, age and gender (Jones et al 1985).
Symptom-limited constant-work-rate test
Endurance exercise capacity was assessed by a symptom-limited constant-work-rate test, which is a reliable and valid method to assess exercise endurance in patients with COPD (van’t Hul et al 2003). This test was performed on the aforementioned cycle-ergometer. After a 2-minute resting period and 3 minutes of unloaded cycling, participants started at ∼70% of their peak external work rate until symptom limitation. At the end of the test Borg symptom scores for dyspnea and fatigue were obtained from all participants.
Blood analyses
Venous blood samples were drawn before the start of both tests, directly at the end of the tests, and 2 and 30 minutes afterwards. They were directly centrifuged and stored at −80 degrees Celsius. Circulating levels of interleukin 6 (IL-6) and interleukin 8 (CXCL8) were determined using Human Inflammation Cytometric Bead Array (Becton Dickinson Biosciences, San Diego, CA, n = 23) (Cook et al 2001). C-reactive protein (CRP) was determined using an immunoturbidimetric assay (Roche Diagnostics Corporation, Indianapolis, USA, n = 30). IGF-I was determined using a radio-immuno assay (n = 30) (van den Berghe et al 2000).
Statistical analyses
Results are presented as median (interquartile range, IQR). A Mann-Whitney U test was used to determine possible differences between physiological characteristics of hospitalized and clinically stable patients with COPD. Moreover, possible changes in systemic levels of IL-6, CXCL8, CRP, and IGF-I following exercise were analyzed by using a general linear models procedure repeated measures analysis of variance, incorporating baseline values as covariates. Spearman rank correlations (r) were used to determine relationships. A priori, a two-sided level of significance was set at p ≤ 0.05 (Altman et al 1983).
Results
Characteristics
On average, patients had moderate-to-severe COPD, normal body mass index, skeletal muscle weakness and reduced functional and peak exercise capacity (Table 1). As compared with the stable patients, hospitalized patients had significantly lower values for the distance walked in 6 minutes (Table 1). On average, both cycling exercise bouts were limited by reaching the maximal voluntary ventilation. High Borg symptom scores for fatigue and dyspnoea confirmed maximal effort (Tables 2 and 3). Oxygen uptake, heart rate and ventilation at the end of the symptom-limited constant-work-rate test were equal to the values obtained at the end of the symptom-limited peak exercise test in both groups (Table 3).
Table 1.
Characteristics
| Whole group | Hospitalized patients | Stable patients | p-value | ||||
|---|---|---|---|---|---|---|---|
| Sex, M : F | 23 | : 7 | 10 | : 4 | 13 | : 3 | – |
| Age, years | 69 | (59–74) | 65 | (59–74) | 73 | (60–75) | 0.43 |
| Height, cm | 165 | (160–171) | 162 | (157–169) | 167 | (163–172) | 0.14 |
| BMI, kg · m−2 | 25.6 | (23.4–29.4) | 25.2 | (22.8–29.4) | 25.8 | (24.9–28.5) | 0.92 |
| FEV1, l | 1.09 | (0.80–1.43) | 1.06 | (0.68–1.37) | 1.18 | (0.81–1.59) | 0.42 |
| FEV1, % reference | 42 | (33–55) | 41 | (33–54) | 45 | (33–58) | 0.47 |
| FEV1/FVC, % | 39 | (35–46) | 38 | (36–45) | 42 | (34–47) | 0.76 |
| TL,CO, mmol kPa−1 min−1 | 3.3 | (2.6–4.5) | 3.0 | (2.6–4.0) | 3.7 | (2.5–4.7) | 0.42 |
| TL,CO, % reference | 42 | (34–55) | 41 | (32–51) | 50 | (35–59) | 0.30 |
| 6MWD, m | 398 | (257–520) | 251 | (204–507) | 439 | (338–554) | 0.04 |
| 6MWD, % reference | 63 | (43–78) | 42 | (37–72) | 72 | (56–85) | 0.02 |
| PImax, % reference | 74 | (58–83) | 67 | (54–79) | 77 | (63–88) | 0.27 |
| QPT, % reference | 72 | (61–89) | 67 | (43–81) | 75 | (66–93) | 0.13 |
Values are expressed as median (interquartile range), except for gender distribution (absolute numbers). Male (M), female (F), meters (m), body mass index (BMI), body weight in kilograms per square height in metres (kg · m−2), forced vital capacity (FVC), litres (l), percent of the reference values (% reference), forced expiratory volume in the first second (FEV1), percent (%), transfer factor for carbon monoxide of the lungs (TL,CO), distance walked in 6 minutes (6MWD), meters (m), maximal inspiratory pressure (PImax), and quadriceps peak torque (QPT). p-value: comparison between hospitalized and stable patients with COPD.
Table 2.
Symptom-limited peak exercise test
| Whole group | Hospitalized patients | Stable patients | p-value | ||||
|---|---|---|---|---|---|---|---|
| Peak load, watts | 67 | (47–100) | 67 | (37–88) | 62 | (52–113) | 0.39 |
| Peak load, % reference | 56 | (39–68) | 50 | (41–62) | 64 | (38–74) | 0.31 |
| Time, s | 325 | (210–550) | 345 | (180–460) | 309 | (245–600) | 0.38 |
| Peak VO2, ml·min−1 | 855 | (670–1201) | 918 | (669–1147) | 802 | (680–1258) | 0.71 |
| Peak VO2, % reference | 51 | (34–71) | 47 | (34–76) | 52 | (35–68) | 0.90 |
| HR, bpm | 129 | (119–147) | 132 | (118–149) | 128 | (121–147) | 0.90 |
| HR, % HRmax calculated | 86 | (79–92) | 88 | (79–92) | 86 | (78–95) | 0.76 |
| VE, l·min−1 | 38.6 | (31.0–54.7) | 43.5 | (23.5–54.7) | 36.3 | (31.8–52.3) | 0.80 |
| VE/MVV, % | 96 | (87–106) | 105 | (90–112) | 94 | (84–99) | 0.10 |
| Borg dyspnea, points | 5 | (5–7) | 5 | (5–9) | 6 | (4–7) | 0.79 |
| Borg fatigue, points | 5 | (4–6) | 4 | (3–5) | 5 | (4–7) | 0.16 |
| Lactate, mmol | 5.71 | (4.25–7.87) | 6.18 | (4.27–7.67) | 5.43 | (3.89–8.06) | 0.66 |
Values are expressed as median (interquartile range). Expressed as a percent of the reference values (% reference), seconds (s), oxygen uptake (VO2), millilitres per minute (ml · min−1), heart rate (HR), beats per minute (bpm), ventilation (VE), litres per minute (l · min–1), and maximum voluntary ventilation (MVV). p-value: comparison between hospitalized and stable patients with COPD.
Table 3.
Symptom-limited constant-work-rate exercise test
| Whole group | Hospitalized patients | Stable patients | p-value | ||||
|---|---|---|---|---|---|---|---|
| Load, watts | 47 | (27–67) | 48 | (27–62) | 42 | (36–72) | 0.43 |
| Load, % peak test | 69 | (63–73) | 71 | (63–73) | 67 | (62–73) | 0.88 |
| Time, s | 455 | (260–880) | 385 | (250–980) | 480 | (270–805) | 0.65 |
| VO2, ml·min−1 | 867 | (659–1202) | 909 | (681–1153) | 845 | (626–1258) | 0.79 |
| VO2, % peak test | 99 | (88–106) | 99 | (86–104) | 97 | (88–107) | 0.92 |
| HR, bpm | 133 | (117–146) | 139 | (126–151) | 131 | (104–143) | 0.23 |
| HR, % peak test | 100 | (93–107) | 102 | (98–109) | 98 | (88–106) | 0.21 |
| VE, l·min−1 | 38.1 | (30.6–55.7) | 44.9 | (25.3–55.7) | 34.9 | (32.4–53.7) | 0.82 |
| VE/MVV, % | 95 | (79–114) | 113 | (92–115) | 90 | (73–95) | 0.02 |
| Borg dyspnoea, points | 5 | (4–7) | 4 | (3–6) | 7 | (4–7) | 0.07 |
| Borg fatigue, points | 5 | (4–7) | 5 | (4–7) | 5 | (4–6) | 0.83 |
| Lactate, mmol | 5.66 | (4.34–7.64) | 6.73 | (5.76–8.82) | 4.86 | (3.90–5.83) | 0.03 |
Values are expressed as median (interquartile range). Expressed as a percent of the reference values (% reference), seconds (s), oxygen uptake (VO2), millilitres per minute (ml · min−1), heart rate (HR), beats per minute (bpm), ventilation (VE), litres per minute (l · min−1), and maximum voluntary ventilation (MVV). P-value: comparison between hospitalized and stable patients with COPD.
As compared to patients who cycled >10 minutes (n = 12), those with an endurance exercise time of ≤10 minutes (n = 18) were older (69 ± 7 versus 62 ± 10 years, p = 0.02), had a significantly worse FEV1 (37 ± 12 versus 57 ± 14% of the reference values, p = 0.0005) and 6-min walking distance (50 ± 18% versus 80 ± 13% of the reference values, p = 0.0001), and tended to be weaker (quadriceps peak torque: 69 ± 29 versus 80 ± 18% of the reference values; and maximal inspiratory mouth pressure: 68 ± 20 versus 82 ± 23% of the reference values, p = 0.08).
Acute anabolic response to exercise tests
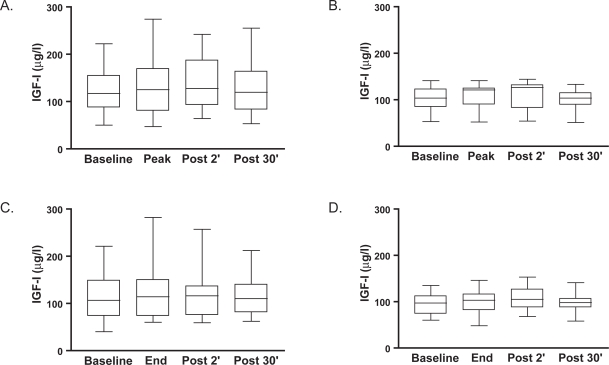
At baseline, median circulating levels of IGF-I were not significantly different between the patients admitted to the hospital [117 μg/l (87 to 149)] and those with stable disease [104 μg/l (84 to 123)]. Median circulating levels of IGF-I increased somewhat between the start of the tests and 2 minutes after ceasing the exercise, directly followed by a reduction to baseline levels 30 minutes after exercise in both tests in both groups (Figure 1). These changes, however, did not reach the level of significance over time or between groups.
Figure 1.
Median (interquartile range) circulating levels of insulin-like growth factor I (IGF-I, expressed in μg/l) at baseline, at the peak and 2 and 30 minutes following a symptom-limited peak exercise test in hospitalized patients with COPD (Panel A) and clinically stable patients with COPD (Panel B). Median (interquartile range) circulating levels of IGF-I at baseline, at the end and 2 and 30 minutes following a symptom-limited constant-work-rate endurance test in hospitalized patients with COPD (Panel C) and clinically stable patients with COPD (Panel D).
Acute inflammatory response to exercise tests
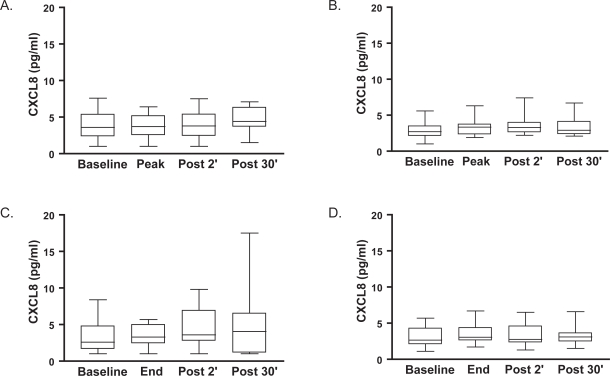
Median baseline circulating levels of CRP and CXCL8 were not different between the patients admitted to the hospital [3.7 mg/l (3.0–35.3) and 3.6 pg/ml (1.5–4.7), respectively] and those with stable disease [3.4 mg/l (3.0–7.2) and 2.7 pg/ml (2.1–3.5), respectively]. Neither median circulating levels of CRP (data not shown), nor circulating levels of CXCL8 changed during or 30 minutes after either type of exercise bout in either group (Figure 2). Baseline systemic IL-6 could only be detected in 22% of the patients, and did not change significantly during or after both exercise tests.
Figure 2.
Median (interquartile range) circulating levels of interleukin 8 (CXCL8, expressed in pg/ml) at baseline, at the peak and 2 and 30 minutes following a symptom-limited peak exercise test in hospitalized patients with COPD (Panel A) and clinically stable patients with COPD (Panel B). Median (interquartile range) circulating levels of CXCL8 at baseline, at the end and 2 and 30 minutes following a symptom-limited constant-work-rate endurance test in hospitalized patients with COPD (Panel C) and clinically stable patients with COPD (Panel D).
Discussion
The main objective of the present study was to compare the acute systemic anabolic and inflammatory responses following two types of exercise bouts in hospitalized patients with COPD with those of patients with clinically stable COPD. In both groups, IGF-I increased non-significantly with ∼10% directly after exercise, but returned to baseline within 30 minutes. No acute inflammatory response was observed.
Acute systemic anabolic response
The somatotrophic axis is one of the major pathways of the endocrine system, which is involved in the protein synthesis and subsequent muscle hypertrophy. The present types of exercise did not result in statistically significant increases in circulating levels of IGF-I (Figure 1). Nevertheless, transient changes in circulating levels of IGF-I during or following high-intensity exercise bouts have been reported (Schwarz et al 1996; Ehrnborg et al 2003). Although the source of this transient release of circulating IGF-I remains unknown, different potential origins can be present. Indeed, this may be a result of an increased release of growth hormone (Dall et al 2000). Nevertheless, the acute exercise-induced increase in circulating levels of IGF-I appears to be independent of the circulating levels of growth hormone and of its response to exercise (Cappon et al 1994; Schwarz et al 1996; Hornum et al 1997). In fact, growth-hormone-releasing factor from the hypothalamus influences resting growth hormone secretion by directly stimulating the anterior pituitary gland. In response to growth hormone stimulation, liver cells synthesize insulin-like growth factors (IGF) I, a process requiring between 8 and 30 hours.
Changes in circulating levels of IGF-I most probably do not reflect possible changes in muscle IGF-I mRNA concentration following exercise (Bamman et al 2001). Therefore, studies including muscle biopsies are necessary to understand to what degree acute transient changes in the circulating IGF-I levels are associated with the anabolic effects of one or more bouts of exercise in patients with COPD.
Inflammatory response
In the present study, no significant increases have been found in the circulating levels of CRP, IL-6 or CXCL8 during or after symptom-limited peak and constant-work-rate exercise bouts. This is in contrast with the results of Rabinovich and colleagues, who found increased systemic inflammation after cycling for 11 minutes at ∼40% of the achieved peak external work rate (circulating levels of tumor necrosis factor-alpha: +6 pg/ml, without increases of circulating levels of IL-6 or soluble TNF receptors) (Rabinovich et al 2003). The reason for this discrepancy is unclear. The latter authors suggested that the increased circulating levels of inflammatory markers are related to the exercise-induced muscle damage and the intensity and duration of the exercise (Rabinovich et al 2003). Exercise bouts at ∼40% of achieved peak external work rate are known to induce muscle damage in patients with COPD (Engelen et al 2001). Unfortunately, in the present study no biopsies of the vastus lateralis muscle were taken. Nevertheless, the present authors expect muscle damage also to be present after exercise bouts at higher intensities (100% and 70% of the peak work load, respectively). Therefore, the duration of the exercise bouts may be the most important factor to induce significant changes in the circulating inflammatory status. Indeed, enormous transient increases in circulating levels of IL-6 and CXCL8 were found 15–30 minutes after prolonged, intense exercise in healthy athletes (Ostrowski et al 2001; Nieman et al 2003). In addition, higher gene expression of several cytokines, of which the increase in muscle CXCL8 (up to 23.3-fold of baseline values) was the largest after running at ∼70% of the peak oxygen uptake for 3 hours (Nieman et al 2003). So, in the present study the exercise duration was probably too short to induce transient changes in the circulating levels of CRP, IL-6 or CXCL8 (median duration peak exercise test: 5.30 min; median duration constant-work-rate exercise test: 7.30 min). On the other hand, 5 minutes of exhaustive local exercise resulted in increased systemic and local oxidative stress in patients with COPD as compared with healthy age-matched controls (Couillard et al 2002, 2003). This topic warrants further investigation.
Clinical implications
High-intensity exercise training appears to be the most efficient treatment-modality to partially restore muscle function, exercise tolerance and health-related quality of life in patients with stable COPD (Casaburi et al 1991; Maltais et al 1996, 1997; Bernard et al 1999; Neder et al 2000; Troosters et al 2000; Spruit et al 2002). The present study showed no acute systemic inflammatory response to high-intensity cycling bouts which were of a relatively short duration in patients with COPD who recently have been admitted to the hospital. Therefore, it appears that the effects of interval-type of exercise training can be studied in patients with COPD who recently have been discharged from the hospital due to an acute exacerbation of COPD. In fact, Man and colleagues (2004) found a favorable effect of exercise training on health-related quality of life and functional exercise capacity in patients with COPD directly after discharge from the hospital as compared to a control group. Although the latter study did not report on the intensity and duration of the various exercises, from the current results it can be concluded that on average it is probably safe to perform interval-type of exercises (Vogiatzis et al 2002) in patients that have recently experienced a severe acute exacerbation of COPD.
Older and physically weaker patients with a forced expiratory volume in the first second of around or less than 40% of the predicted values, are not likely to be able to sustain high-intensity endurance-type of exercises. Interval training, resistance training and/or transcutaneous neuromuscular electrical stimulation should be considered in these patients (Simpson et al 1992; Coppoolse et al 1999; Bourjeily-Habr et al 2002; Neder et al 2002; Spruit et al 2002; Vogiatzis et al 2002).
Limitations of the study
At first sight, it is somewhat surprising that the hospitalized patients and the clinically stable patients had a similar peak exercise tolerance (Table 2) and a significantly different functional exercise capacity eg, the distance walked in 6 minutes (Table 1). Nonetheless, peak exercise capacity has shown be related to pulmonary function impairment (especially tranfer factor for carbon monoxide) (Gosselink et al 1996), which was similar between the two groups (Table 1). On the other hand, the distance walked in 6 minutes has shown to be more related to the quadriceps muscle weakness in patients with COPD (Gosselink et al 1996), which tended to be significantly different between the two patient groups in the present study.
The severity of the exacerbation may be questioned because hospitalized patients had similar pulmonary function and body mass index as the clinically stable patients (Table 1). Nonetheless, the characteristics and skeletal muscle force of the hospitalized patients were not significantly different from a previous unrelated study (Spruit et al 2003). In contrast, the present clinically stable patients appear to have worse physical characteristics as seen previously (Spruit et al 2003). This is probably because the current patients were free of an acute exacerbation for at least three months, while previously this period was set at 1 year (Spruit et al 2003). Nevertheless, this indicates again that also patients with clinically stable COPD that attend the outpatient consultation of a tertiary care setting are particularly in need of pulmonary rehabilitation (Spruit et al 2004).
The present authors were interested in the acute systemic inflammatory response following a symptom-limited peak exercise test and constant-work-rate exercise test in hospitalized patients who were about to be discharged from the hospital. In the present setting, patients are admitted for about 10 days. Therefore, the symptom-limited peak exercise test had to be done at day 8. Moreover, the patients that Man and colleagues included in their rehabilitation study were also tested after a mean hospital stay of 8 days (Man et al 2004). Whether and to what extent exercise-induced increases will occur in systemic inflammation earlier during the acute COPD exacerbation remains unknown. In addition, by only taking blood samples up to 30 minutes after ceasing the exercise tests, the present study may have missed a later inflammatory response. In fact, important changes in inflammatory gene transcription may result from exercise but would probably not have been detected within 30 minutes. Nonetheless, circulating levels of CXCL8 increased significantly following prolonged high-intensity exercise in healthy athletes, with a peak at 30 minutes (Ostrowski et al 2001).
Finally, the present study did not assess body composition, which recently has been shown to partially influence the exercise-induced systemic inflammatory response in COPD (Van Helvoort et al 2006).
Conclusion
Patients with stable COPD have a reduced physical function (Gosselink et al 1996), which deteriorates during an acute exacerbation of COPD (Spruit et al 2003). Therefore, the latter patients have a clear indication to start exercise training following an acute COPD exacerbation. The present study has shown that hospital admitted patients have similar acute systemic responses to high-intensity exercise cycling as compared to the responses of patients with stable disease. Thus, high-intensity cycling exercises for 10 minutes or less seem feasible in patients directly after an acute exacerbation. The present study adds evidence that changes in several inflammatory markers do not occur with exercise in COPD irrespective of their clinical stability.
Acknowledgments
The authors thank Dr. Peter Bogaerts for drawing all the blood samples and for supervising all the cycling exercise tests; Rik Cuppens for technical support; physiotherapists Veronica Barbier and Iris Coosemans for functional testing; and physiotherapists Maarten Kok and Alex Lambalk for collecting the data.
Abbreviations
- 6MWD
6-minute walking distance
- cm
centimeters
- COPD
chronic obstructive pulmonary disease
- CXCL8
interleukin 8
- F
female
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- HR
heart rate
- IGF-I
insulin-like growth factor one
- IL-6
interleukin 6
- IQR
interquartile range
- kg
kilogram
- M
male
- m
meters
- MVV
maximal voluntary ventilation
- PImax
maximal inspiratory mouth pressure
- QPT
quadriceps peak torque
- s
seconds
- VE
ventilation
- VO2
oxygen uptake
Footnotes
The institution at which the work was performed
Respiratory Rehabilitation and Respiratory Division, University Hospital Gasthuisberg, Leuven, Belgium.
Sources of financial support
The present study was supported by Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen, Levenslijn grant #7.0007.00. Martijn A. Spruit was a post-doctoral fellow at the K.U. Leuven (PDM/04/230). Thierry Troosters is a post-doctoral fellow of the FWO Vlaanderen.
Conflict of interest
All authors state that they do not have a direct financial interest in the subject of the present manuscript.
References
- Anon World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–6. [PubMed] [Google Scholar]
- Altman DG, Gore SM, Gardner MJ, et al. Statistical guidelines for contributors to medical journals. Br Med J (Clin Res Ed) 1983;286:1489–93. doi: 10.1136/bmj.286.6376.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Shipp JR, Jiang J, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–90. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- Bernard S, Whittom F, LeBlanc P, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- Borg GAV. Borg’s perceived exertion and pain scales. 1st edn. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- Bourjeily-Habr G, Rochester CL, Palermo F, et al. Randomised controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax. 2002;57:1045–9. doi: 10.1136/thorax.57.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon J, Brasel JA, Mohan S, et al. Effect of brief exercise on circulating insulin-like growth factor I. J Appl Physiol. 1994;76:2490–6. doi: 10.1152/jappl.1994.76.6.2490. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- Cook EB, Stahl JL, Lowe L, et al. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs non-allergics. J Immunol Methods. 2001;254:109–18. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- Coppoolse R, Schols AM, Baarends EM, et al. Interval versus continuous training in patients with severe COPD: a randomized clinical trial. Eur Respir J. 1999;14:258–63. doi: 10.1034/j.1399-3003.1999.14b04.x. [DOI] [PubMed] [Google Scholar]
- Couillard A, Koechlin C, Cristol JP, et al. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20:1123–9. doi: 10.1183/09031936.02.00014302. [DOI] [PubMed] [Google Scholar]
- Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in COPD patients. Am J Respir Crit Care Med. 2003 doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, et al. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1239–45. doi: 10.1164/ajrccm.162.4.9912016. [DOI] [PubMed] [Google Scholar]
- Dall R, Longobardi S, Ehrnborg C, et al. The effect of four weeks of supraphysiological growth hormone administration on the insulin-like growth factor axis in women and men. GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:4193–200. doi: 10.1210/jcem.85.11.6964. [DOI] [PubMed] [Google Scholar]
- Debigare R, Cote CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med. 2001;164:1712–17. doi: 10.1164/ajrccm.164.9.2104035. [DOI] [PubMed] [Google Scholar]
- Debigare R, Marquis K, Cote CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124:83–9. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- Dentener MA, Creutzberg EC, Schols AM, et al. Systemic anti-inflammatory mediators in COPD: increase in soluble inter-leukin 1 receptor II during treatment of exacerbations. Thorax. 2001;56:721–6. doi: 10.1136/thorax.56.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnborg C, Lange KH, Dall R, et al. The growth hormone/insulin-like growth factor-I axis hormones and bone markers in elite athletes in response to a maximum exercise test. J Clin Endocrinol Metab. 2003;88:394–401. doi: 10.1210/jc.2002-020037. [DOI] [PubMed] [Google Scholar]
- Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–18. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- Engelen MP, Wouters EF, Deutz NE, et al. Effects of exercise on amino acid metabolism in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:859–64. doi: 10.1164/ajrccm.163.4.2006137. [DOI] [PubMed] [Google Scholar]
- Fernandez-Celemin L, Pasko N, Blomart V, et al. Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2002;283:E1279–90. doi: 10.1152/ajpendo.00054.2002. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–80. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- Hornum M, Cooper DM, Brasel JA, et al. Exercise-induced changes in circulating growth factors with cyclic variation in plasma estradiol in women. J Appl Physiol. 1997;82:1946–51. doi: 10.1152/jappl.1997.82.6.1946. [DOI] [PubMed] [Google Scholar]
- Jones NL, Makrides L, Hitchcock C, et al. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–8. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:555–61. doi: 10.1164/ajrccm.155.2.9032194. [DOI] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:442–7. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ. 2004;329:1209. doi: 10.1136/bmj.38258.662720.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neder JA, Jones PW, Nery LE, et al. Determinants of the exercise endurance capacity in patients with chronic obstructive pulmonary disease. The power-duration relationship. Am J Respir Crit Care Med. 2000;162:497–504. doi: 10.1164/ajrccm.162.2.9907122. [DOI] [PubMed] [Google Scholar]
- Neder JA, Sword D, Ward SA, et al. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD) Thorax. 2002;57:333–7. doi: 10.1136/thorax.57.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet D, Oh Y, Kim HS, et al. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–9. doi: 10.1542/peds.110.4.681. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–25. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Asp S, et al. Chemokines are elevated in plasma after strenuous exercise in humans. Eur J Appl Physiol. 2001;84:244–5. doi: 10.1007/s004210170012. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans – effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–15. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- Rabinovich RA, Figueras M, Ardite E, et al. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21:789–94. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5 Suppl 2):398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Brasel JA, Hintz RL, et al. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–7. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- Simpson K, Killian K, McCartney N, et al. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax. 1992;47:70–5. doi: 10.1136/thx.47.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruit MA, Gosselink R, Troosters T, et al. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J. 2002;19:1072–8. doi: 10.1183/09031936.02.00287102. [DOI] [PubMed] [Google Scholar]
- Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58:752–6. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruit MA, Troosters T, Trappenburg JCA, et al. Exercise training during rehabilitation of patients with COPD: a current perspective. Patient Educ Couns. 2004;52:243–8. doi: 10.1016/S0738-3991(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Tirakitsoontorn P, Nussbaum E, Moser C, et al. Fitness, acute exercise, and anabolic and catabolic mediators in cystic fibrosis. Am J Respir Crit Care Med. 2001;164:1432–7. doi: 10.1164/ajrccm.164.8.2102045. [DOI] [PubMed] [Google Scholar]
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–12. doi: 10.1016/s0002-9343(00)00472-1. [DOI] [PubMed] [Google Scholar]
- van’t Hul A, Gosselink R, Kwakkel G. Constant-load cycle endurance performance: test-retest reliability and validity in patients with COPD. J Cardiopulm Rehabil. 2003;23:143–50. doi: 10.1097/00008483-200303000-00012. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Baxter RC, Weekers F, et al. A paradoxical gender dissociation within the growth hormone/insulin-like growth factor I axis during protracted critical illness. J Clin Endocrinol Metab. 2000;85:183–92. doi: 10.1210/jcem.85.1.6316. [DOI] [PubMed] [Google Scholar]
- Van Helvoort HA, Heijdra YF, Thijs HM, et al. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc. 2006;38:1543–52. doi: 10.1249/01.mss.0000228331.13123.53. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J. 2002;20:12–19. doi: 10.1183/09031936.02.01152001. [DOI] [PubMed] [Google Scholar]
- Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–15. [PubMed] [Google Scholar]