Table 5.
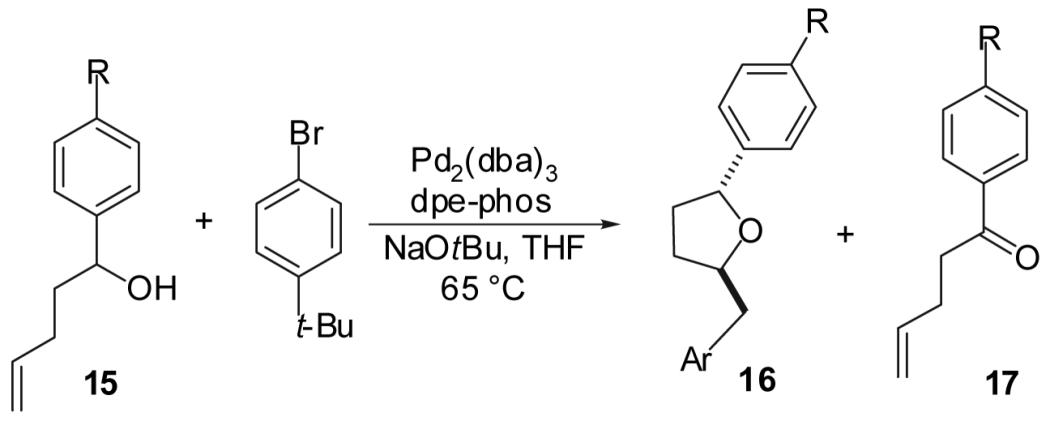 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | R | Product | Yieldb | dr | 16:17c |
| 1 | 15a | CN | 16a | 76% | >20:1 | >20:1 |
| 2 | 15b | CF3 | 16b | 64% | >20:1 | >20:1 |
| 3 | 11e | H | 16c | 69% | >20:1 | >20:1 |
| 4 | 15c | OMe | 16d | 58% | >20:1 | 5:1 |
| 5 | 15d | N(Me)2 | 16e | 35% | >20:1 | 3:1 |
(a) Conditions: 1.0 equiv alcohol, 2.0 equiv ArBr, 2.0 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % dpe-phos, THF (0.13-0.25 M), 65 °C.
(b) Yields represent average isolated yields for two or more experiments.
(c) The formation of t-butylbenzene in amounts comparable to the amount of 17 observed in these reactions was detected by GC analysis.
