Table 9.
| Entry | Alcohol | R2-Br | Product | Yield(b) | dr |
|---|---|---|---|---|---|
| 1 | 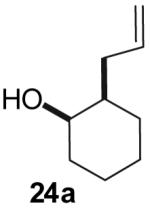 |
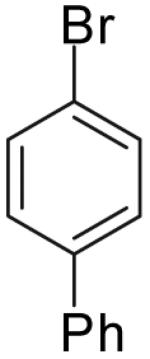 |
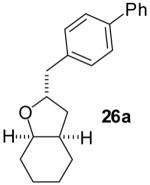 |
60% | 10:1 |
| 2 | 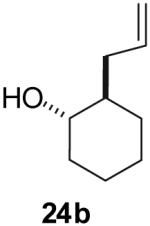 |
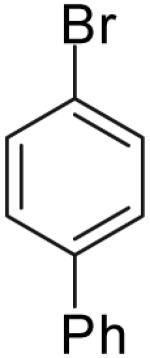 |
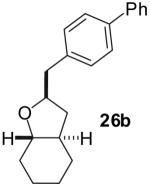 |
70% | >20:1 |
| 3 | 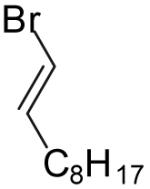 |
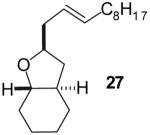 |
40% | >20:1 | |
| 4 | 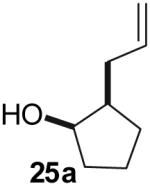 |
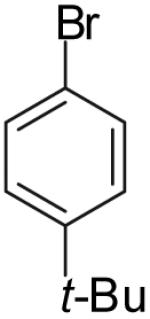 |
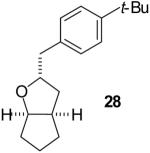 |
53% | >20:1 |
| 5(a)(b) | 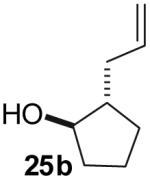 |
 |
0%(c) | -- |
Conditions: 1.0 equiv alcohol, 2.0 equiv ArBr, 2.0 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % dpe-phos, THF (0.13-0.25 M), 65 °C.
Yields represent average isolated yields for two or more experiments.
Oxidation of the alcohol substrate to 2-allylcyclopentanone was observed.
