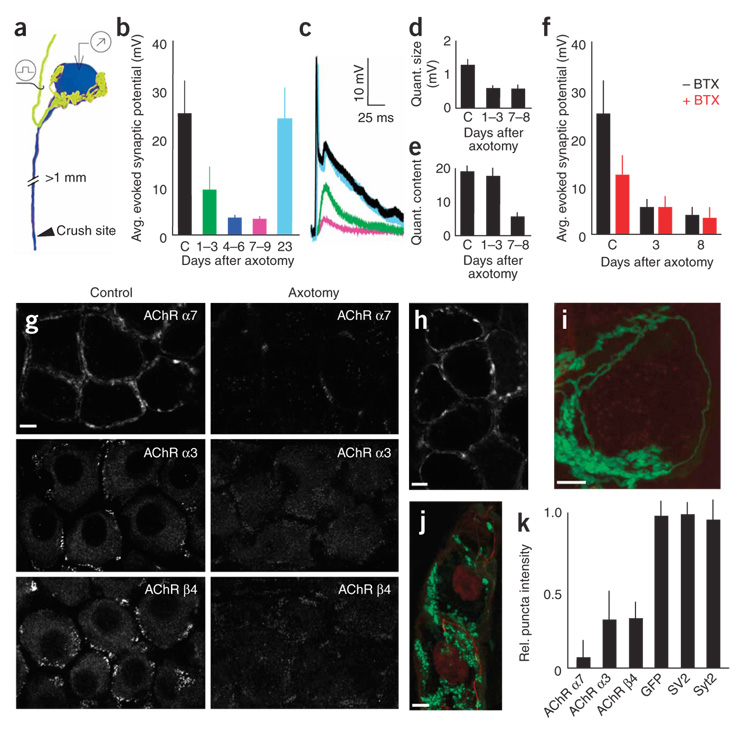
Figure 5. Postsynaptic AChR loss underlies axotomy-induced synaptic depression.
(a) Schematic depicting the assay for the effects of postganglionic nerve crush on pre- to postganglionic connections (yellow, preganglionic axon; blue: postganglionic neuron). (b–f) Physiological analysis of axotomized SMG neurons (c shows control values from normal cells). The amplitudes of evoked synaptic potentials were rapidly reduced after axotomy in the SMG (b). Also shown are sample traces from control and axotomized neurons (c); colors correspond to those of the datasets shown in b. Quantal size was reduced soon after axotomy (d), whereas quantal content was reduced later (e). (f) BTX blocked synaptic transmission in control cells (by ~50%) but had no effect on cells that had been axotomized 3–8 d before. (g) 72 h after nerve crushing, AChR α7 (BTX) and α3 and β4 AChR subunits (antibodies) were significantly reduced. (h) Three weeks after axotomy, synaptic transmission recovered and AChR α7 labeling was again visible. (i) 72 h after nerve crush, GFP-labeled preganglionic axons (green) formed normal-appearing basket-like inputs to axotomized ganglion cells even after BTX-labeled AChRs (red) were no longer present. (j) The presynaptic marker synaptotagmin-2 (green) and the axonal marker neurofilament (red) remained in preganglionic axon terminals 72 h after postganglionic axotomy. Scale bars, 5 µm in all images. (k) At 72 h, presynaptic markers were unaffected by axotomy, but postsynaptic AChRs were significantly reduced. The intensity of synaptic puncta staining in axotomy was compared to controls to give a relative measure. Error bars, s.e.m.