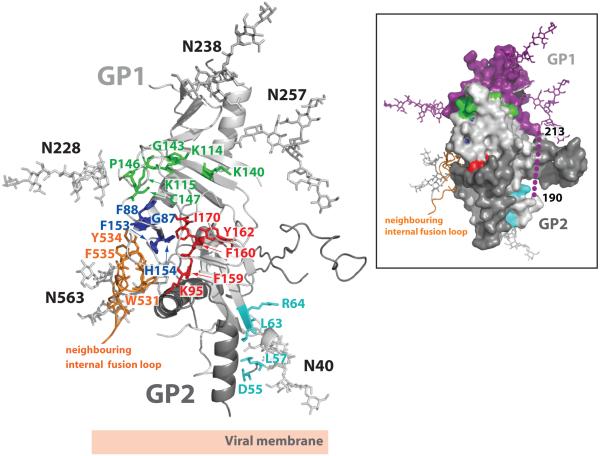
Figure 5. Sites of receptor binding and cathepsin cleavage.
Residues coloured in cyan, green, royal blue and red were previously identified by mutagenesis to be important for viral entry48-50. Residues coloured in cyan (D55, L57, L63 and R64) reside at the base of the chalice, near the GP1-GP2 disulfide bond and HR1D, and are likely important for fusion-mediated conformational changes rather than receptor binding. Residues coloured in red (F159, F160, Y162 and I170) are primarily buried hydrophobic amino acids that help to maintain the structural stability of GP1. Residues coloured in royal blue (G87, F88, F153 and H154) are in proximity to the putative receptor-binding site (RBS) and pack against the hydrophobic residues from a neighbouring internal fusion loop (coloured orange). Mutations to these residues may affect viral entry by altering the structural integrity of the RBS and/or by affecting packing of the fusion loop. Residues coloured in green (K114, K115, K140, G143, P146 and C147) reside on or near the GP surface and may contribute to receptor binding. A molecular surface representation of a GP monomer, coloured and oriented according to the ribbon diagram, is presented in the inset box. Indicated in this view: cleavage at a site on the loop (shown as purple dots) between residues 190-213 would remove the entire glycan cap (coloured in purple) and the mucin-like domain (not shown), leaving GP2 and an ∼18 kDa fragment of GP1.