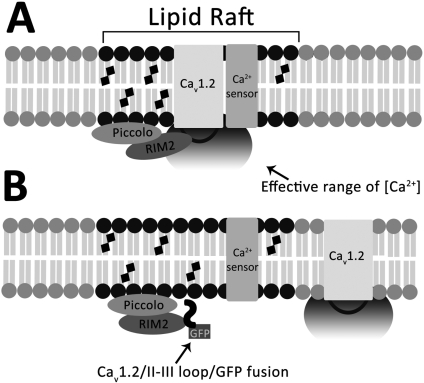
Fig. 8.
Model for inhibition of GLP-1 potentiation of GSIS by displacement of Cav1.2 or Cav1.3 from lipid rafts. A, intracellular interdomain II-III loops of Cav1.2 and 1.3 direct localization of the respective channels to lipid rafts via binding to raft-resident proteins. In Cav1.2, binding to the raft resident RIM2 anchors the channel in the lipid raft domain. A similar interaction with a yet unidentified protein is proposed to anchor Cav1.3 to lipid rafts. Other proteins required for insulin exocytosis or extracellular signal-regulated kinase 1/2 phosphorylation are also localized to lipid rafts. B, overexpression of the Cav1.2/II-III loop/GFP fusion peptide in INS-1 cells competitively displaces the endogenous Cav1.2 channel from lipid rafts by binding to raft resident protein RIM2, spatially uncoupling them from Ca2+-dependent processes such as GLP-1 potentiation of GSIS. Augmentation of Ca2+ influx via the displaced channels with the L-type channel agonist FPL-64176 can partially compensate for the increased distance between channel pore and Ca2+-sensing proteins involved in secretion (see Fig. 1C).