Figure 6. CDKN1A/p21 expression levels are regulated by MAPK/ERK in V-400R2 cells.
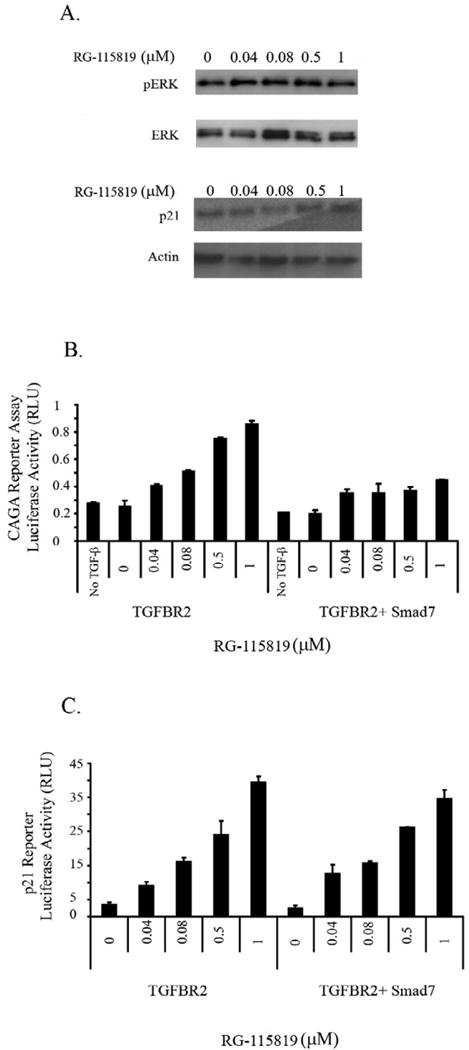
The V-400R2 cell line was cultured in the presence of RG-115819, TGF-β (10ng/ml) and the MAPK/ERK inhibitor U0126 for 48 hours. A. No increase in phosphorylated ERK (pERK) or in CDKN1A/p21 expression is detected in V-400R2 cells treated with the MAPK/ERK inhibitor demonstrating CDKN1A/p21's expression is regulated by ERK in a TGFBR2 dependent manner. The activation of ERK is considerably reduced due to the presence of the inhibitor. Of note, total levels of ERK were not altered. CDKN1A/p21 and pERK are detectable with vehicle only treatment, which is likely a consequence of a nonspecific effect of the U0126 vehicle. B. Inhibition of TGF-β mediated 3TPLux reporter activity by Smad7 occurs with all concentrations of ligand. V-400R2 cells were transfected with a plasmid expressing Smad7 in order to inhibit the Smad dependent pathway, and then transfected with the 3TP-Lux reporter. The dose dependent relation between luciferase activity and TGFBR2 is blocked by Smad7 demonstrating Smad7 is blocking Smad mediated transcription. C. Smad7 does not inhibit TGFBR2 dependent increases in CDKN1A/p21 luciferase reporter activity. V-400R2 cells were transfected with both Smad7 and the CDKN1A/p21 reporter assay. Interestingly, no significant changes in the activity of the CDKN1A/p21 luciferase reporter is detected after Smad7 transfection demonstrating that the expression level of TGFBR2 does not regulate CDKN1A/p21 expression through Smad signaling. The intensity of the bands was determined using densitometry as described in the prior figure legends. Densitometry for phosphorylated ERK revealed the following values: 120 (0 μM), 130 (0.04 μM), 126 (0.08μM), 145 (0.5 μM) and 131 (1 μM) and for p21: 130 (0 μM), 135 (0.04 μM), 142 (0.08 μM), 142 (0.5 μM) and 145 (1 μM).
