Abstract
The aim of this study was to investigate the reliability of a new classification system for low back-related leg pain arising from neural tissue dysfunction. Leg pain is a frequent accompaniment to back pain and is an indicator of the severity and prognosis of the disorder. For optimal patient care, treatment should be directed according to the identified pathophysiological mechanisms. The authors have proposed a sub-classification of neural low back-related leg pain into four categories, each requiring a different management strategy: Central Sensitization (CS), comprising major features of sensitization of the somatosensory system; Denervation (D), arising from significant axonal compromise without evidence of major central nervous system changes; Peripheral Nerve Sensitization (PNS), arising from nerve trunk inflammation without clinical evidence of significant denervation; and Musculoskeletal pain (M), referred from non-neural structures such as the disc or facet joints. The purpose of this study was to investigate the interrater reliability of this classification system. Forty consecutive patients with unilateral low back-related leg pain were independently assessed by five pairs of examiners using a physical examination protocol, screening for central sensitization of the somatosensory system, neurological deficit, and nerve tissue mechano-sensitization. Subjects were classified as follows: CS 30%, D 27.5%, PNS 10%, and M 32.5%. Interrater reliability was good with 80% agreement and a k of 0.72 (95% Confidence Interval (CI) .57–.86). The findings of the study demonstrate that patients with low back-related leg pain can be reliably classified to one of the four proposed groups.
KEYWORDS: Classification, Interrater Reliability, Leg Pain, Low Back Pain
In 1997 the importance of identifying specific and effective treatment in prisubgroups of low back pain (LBP) was mary care and to determine which treat-given top ranking by the Second In-ments will be most effective in clinical ternational Forum for Primary Care Re-research. Ten years later, the same issue is search on Low Back Pain1. Classification still seen as the number-one research priof “non-specific” LBP patients into dis-ority by primary care practitioners2. tinct subgroups is essential to deliver There is considerable controversy in the literature regarding correct diagnosis and classification3,4, in particular for low back-related leg pain, which is associated with LBP in up to 65% of cases5–8.
Traditionally, a distinction has been made between radicular pain and somatic referred pain9, but in the absence of a true diagnostic gold standard, the differentiation between the two remains difficult10. Failure to correctly classify subjects into homogenous subgroups may be one explanation for inconsistent findings of randomized controlled trials investigating the effectiveness of conservative treatment of patients with sciatica11,12. This is in spite of the fact that a number of classification systems for LBP incorporating radiating leg pain have been published since 198113–15. Current studies have demonstrated that conservative therapy, when implementing classification systems, can be effective for two subgroups of patients with LBP and lower limb symptoms16–19. Patients with low back-related leg pain without neurological signs, whose symptoms centralize with repeated movement in a specific direction (directional preference), have shown to improve with direction-specific treatment16,18,19. Furthermore, patients with lower limb symptoms, signs of nerve root compression, and either a crossed straight leg raise or peripheralization of symptoms with extension movements appear to benefit from a traction-based treatment intervention17.
The categorization of these two groups of patients with low back-related leg pain arises from a treatment-based classification system for LBP14. however, there is ample evidence in the literature suggesting the existence of at least two further subgroups with similar symptoms but different patho-mechanisms. hence, our classification system can be seen as an extension of existing classification systems by adding one further dimension for neural-related pain. One subgroup consists of patients with pain arising from a nerve lesion affecting the somatosensory system, resulting in sensory hypersensitivity, closely connected to central pain mechanisms20,21. One other subgroup consists of patients with mechano-sensitization of the nerve trunk due to inflammatory processes in and around the peripheral nerve22–24.
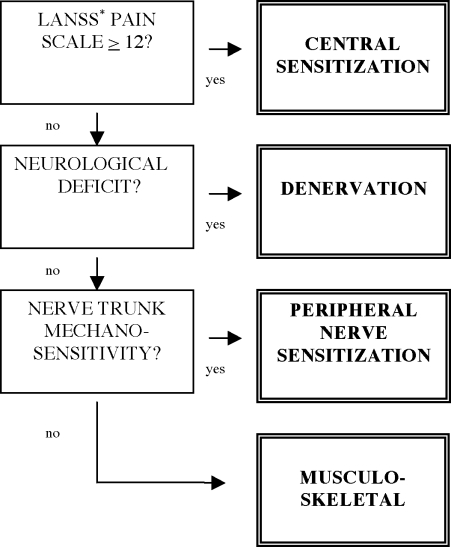
It has been suggested that for the more complex pain conditions related to nerve injury, a classification based on patho-mechanisms may be more suitable25,26 as it offers a rational approach to treatment and greater diagnostic sensitivity, and it has the potential to provide information about the prognosis and natural course of the disorder26. Therefore, we developed a patho-mechanismbased classification system for low back-related leg pain27, extending the ideas originally presented by Elvey and hall28. The purpose of refining this classification system was to improve treatment outcome, particularly with respect to identifying patients most likely to respond to neural mobilization. Depending on the assumed predominance of patho-mechanisms, low back-related leg pain is differentiated into four distinct categories. Classified hierarchically, these categories are Central Sensitization (CS), comprising major features of sensory sensitization; Denervation (D), arising from significant axonal compromise without major evidence of the above; Peripheral Nerve Sensitization (PNS), arising from nerve trunk inflammation without clinical evidence of significant denervation; and Musculoskeletal pain (M), referred from non-neural structures such as the lumbar intervertebral disc or zygapophyseal joints27 (Figure 1).
FIGURE 1.
Classification algorithm
∗LANSS: Leeds Assessment of Neuropathic Symptoms and Signs42
The category PNS has been previously described28,29 and is thought to characterize patients who may respond to manual therapy involving neural mobilization. There is reasonable evidence showing that patients with signs and symptoms corresponding to PNS benefit from a neural mobilization treatment in the upper quarter30–33 and limited evidence for a response in the lower quarter34,35. In contrast, patients classified as D might need a different treatment approach as it seems unlikely that structural nerve damage will respond favorably to neural mobilization. The effects of neural mobilization treatment in a patient with lumbar radiculopathy with signs and symptoms corresponding to group D have been investigated in a case report; no treatment benefits were demonstrated36. Additionally, the effects of a neural mobilization intervention on pain and disability have been investigated in patients following decompression surgery37. Patients referred for spinal decompression surgery are likely to have significant evidence of nerve root compression, with corresponding sensory and motor deficit, consistent with group D. The results of that study indicated no beneficial effect in favor of the neural mobilization. however, patients with lower limb symptoms and signs of nerve root compression appear to benefit from a traction-based treatment intervention17.
Patients classified in group CS have overriding sensory hypersensitivity generated in part by central pain mechanisms, which is unlikely to respond to manual therapy, as has been shown in patients with whiplash-associated disorders38. Patients classified as CS may need to be referred to multimodal treatment programs involving cognitive restructuring and pain medication39.
Finally, patients in group M with somatic referred leg pain are unlikely to benefit from a treatment approach directed at neural structures in the lumbar spine. These patients would potentially benefit from an intervention derived from a treatment-based classification system16,18,19.
These examples show that a classification system is not only crucial in order to provide the patients with an explanatory rationale for their disorder but also to select treatments that interact with specific mechanisms40. Systems of classification must result in consistent discrimination between groups in order to be valid41. The aim of the present study was therefore to investigate the interrater reliability of a new classification system for neural (low back)-related leg pain27 as the first in a series of studies designed to evaluate the validity of the system.
Methods
This study was approved by human Research Ethics Committee, Curtin University of Technology, Perth, Western Australia. Data were collected between February 2006 and July 2007. All subjects provided written informed consent.
Subjects
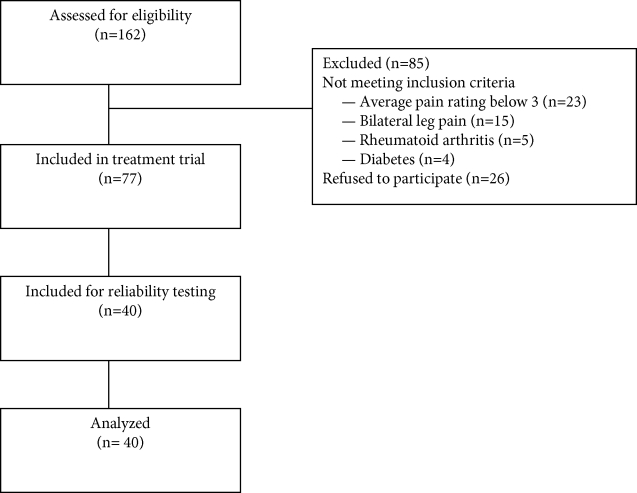
Data for this study were collected from 40 of 77 subjects participating in another study (Figure 2). They were recruited from 162 consecutive patients with low back-related leg pain referred to the physiotherapy department at a private multidisciplinary pain clinic. Patients were referred from within the pain clinic, two orthopedic private practices, and a neurosurgery private practice in hamburg, Germany. Of the patients referred, 26 were not interested in participating. Those willing to participate were screened for eligibility according to strict inclusion and exclusion criteria. Inclusion criteria were presence of unilateral leg pain of more than 6 weeks and a pain score on the 11-point numerical rating scale of more than 3. Patients were excluded if they fulfilled one of the following criteria: 1) history of lower quadrant surgery or trauma within the past 6 months, 2) nerve root block within the past 4 weeks, 3) history of neuropathic pathology such as diabetes or polyneuropathies, 4) history of vascular disease in the lower extremities, 5) systemic disease, 6) inflammatory arthropathies, 7) contraindications to manual therapy techniques, and 8) inability to understand written/spoken German. Forty-seven subjects did not meet the selection criteria. Subjective demographic details are shown in Table 1 and data from the physical examination in Table 2.
FIGURE 2.
Flow diagram
TABLE 1.
Subjective characteristics
| Mean (SD) | |
|---|---|
| Number (n) | 40 |
| Age | 47.8 (13.1) |
| Gender (% male) | 40 |
| Pain below knee (%) | 76.3 |
| Pain duration current episode in months (mean/interquartile range) | 7.5 (4.0) |
| Present pain (NRS) | 5.0 (1.6) |
| LANSS score∗ | 8.1 (5.7) |
| RMDQ | 8.0 (4.4) |
| FABQ | 34.7 (19.1) |
| hADS-A | 6.8 (4.1) |
| hADS-D | 5.3 (3.6) |
NRS = 11-point numerical rating scale: 0 = no pain to 10 = worst imaginable pain; the score was the mean of three NRS scores: present pain, worst pain, and least pain in the last seven days.
LANSS = Leeds Assessment of Neuropathic Symptoms and Signs
RMDQ = Roland Morris Disability Questionnaire score
FABQ = Fear Avoidance Questionnaire
hADS-A = hospital Anxiety and Depression Scale—subscale anxiety
hADS-D = hospital Anxiety and Depression Scale—subscale depression
SD = standard deviation
TABLE 2.
Objective characteristics
| CS | D | PNS | M | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Refex defcit | 4 | 33 | 4 | 33 | 0 | 0 | 1 | 8 | 9 | 23 |
| Strength defcit | 6 | 50 | 6 | 50 | 0 | 0 | 5 | 41 | 17 | 43 |
| PP defcit | 8 | 67 | 9 | 75 | 0 | 0 | 4 | 33 | 21 | 53 |
| LT defcit | 6 | 50 | 7 | 58 | 1 | 25 | 1 | 8 | 15 | 38 |
| Positive NP | 10 | 83 | 9 | 75 | 4 | 100 | 3 | 27 | 26 | 67 |
| Positive SLR | 3 | 25 | 4 | 33 | 4 | 100 | 4 | 36 | 15 | 39 |
| Crossed SLR | 0 | 0 | 1 | 9 | 0 | 0 | 0 | 0 | 1 | 3 |
| Positive AROMF | 5 | 42 | 4 | 36 | 4 | 100 | 0 | 0 | 13 | 33 |
PP = pinprick; LT = light touch; NP = nerve palpation; SLR = straight leg raise test; AROMF = active range of motion in fexion test; CS = central sensitization; D = denervation; PNS = peripheral nerve sensitization; M = musculoskeletal
Examiners
Six experienced physiotherapists who had a mean (SD) of 10 (2.4) years experience in treating musculoskeletal disorders were the examiners for the study. All had undertaken postgraduate education in manual therapy. To standardize the examination procedure, all examiners attended two group and one individual training sessions prior to the start of the study. Additionally, each examiner was provided with a booklet with photos and a description of each of the tests.
Procedure
Each subject was examined on two occasions, once by AS and once by one of the five other participating physiotherapists. Of the five physiotherapists, one examined 12 subjects, two examined 10 subjects, and two examined 4 subjects. Order of testing varied but could not be randomized due to organizational constraints. All subjects were re-examined within 4 days with 70% of the subjects examined twice within a time frame of 48 hours. Each physiotherapist conducted a complete assessment and recorded the findings in a private treatment room. No information regarding the examination or classification of subjects was exchanged between the examiners. Patients were not informed about findings of the first examination to avoid biasing the second examination.
The examination protocol commenced with a subjective examination including questions relating to area of pain, duration of symptoms. and the questionnaire component of the Leeds Assessment for Neuropathic Symptoms and Signs (LANSS)42. The physical examination incorporated a) a standard neurological examination screening for motor and sensory deficits; b) the objective part of the LANSS assessment, to examine for allodynia and hyperalgesia42; and c) tests for peripheral nerve sensitization43. The examination took 30 minutes per subject, including documentation of test results. The individual tests are described in Appendix A. Patients with a LANSS scale score of 12 or higher were classified as CS. Subjects with LANSS score of less than 12 and evidence of frank nerve root damage leading to negative symptoms and signs such as hypoesthesia or failure of motor units were classified as D. Where the first two categories did not apply and there were signs and symptoms indicative of increased peripheral nerve mechanosensitivity, patients were classified PNS (Appendix A). Finally, subjects who did not meet the previous criteria were classified as M (Figure 1). Prevalence of classifications was calculated from the results of the first examiner.
Sample Size
The sample size estimates were based on the assumption that 10% of patients would be classified as CS, 10% D, 30% PNS, and 50% M. In this case, to achieve a significant result (p<0.05) with a power of above 80%, a sample of 40 subjects measured twice would be sufficient to determine that reliability was at least good (k > 0.6)44,45.
Data Analysis
Data was analyzed using SPSS version 13.0. Percentage agreement, kappa-coefficient (k), and 95% confidence interval (CI) were calculated to determine the level of agreement between examiners46.
Results
There was good agreement between examiners (percentage agreement = 80; k = 0 .72; 95% CI = 0.57 to 0.86). The pro-portion of subjects in each classification differed somewhat between examiners as would be expected where agreement was not 100%. Prevalence of the CS, D, and M groups were comparable (27.5%, 32.5%, and 30%, respectively) with a considerably lower prevalence of PNS (10%) (Table 3). Post hoc power analysis showed a power of 88% for detecting a k of 0.7 with the distribution of groups as described above.
TABLE 3.
Comparison of findings between physiotherapist examiners
| Second Examiner | |||||||
|---|---|---|---|---|---|---|---|
| Central Sensitization | Denervation | Peripheral Nerve Sensitization | Musculo-skeletal | Total | % | ||
| First Examiner | |||||||
| Central Sensitization | 10 | 2 | 0 | 0 | 12 | 30 | |
| Denervation | 0 | 9 | 0 | 2 | 11 | 27.5 | |
| Peripheral Nerve Sensitization | 0 | 1 | 2 | 1 | 4 | 10 | |
| Musculoskeletal | 0 | 2 | 0 | 11 | 13 | 32.5 | |
| Total | 10 | 14 | 2 | 14 | 40 | 100 | |
Discussion
Patients with low back-related leg pain can be reliably classified into four groups by experienced musculoskeletal physiotherapists using a new classification system27 (Figure 1). Reliability is an essential requirement for any valid classification system. Furthermore, to have clinical utility, research results must be generalizable to the clinical environment. Our study was conducted under clinical conditions, with 6 independent examiners assessing consecutive patients with low back-related leg pain. The sample of 40 consecutively recruited subjects (described in Table 1 and 2) can be considered representative of everyday physiotherapy practice in Germany.
Advantages of this classification system are that the classification algorithm relies on routine testing procedures. Experienced clinicians can quickly learn the examination protocol, and the classification algorithm is easy to apply, based on objective decisions for classification into the mutually exclusive categories (for details, see Appendix A). The combination of dichotomous (present/not present) and continuous (e.g., range of motion) measures of outcome minimizes subjective individual interpretations, leading to a clear and easy-to-follow system with good reliability. Thus, clinicians can apply the classification system to their patients without having to learn a new skill-set. Furthermore, they can be confident of the reliability of the classification system when used in the manner described. We believe that the new classification system we are evaluating can be combined with previously reported systems of classifying LBP13–15. It was not our intention to replace these, simply to add to them. The purpose of refining these classification systems is to more accurately differentiate neural-related disorders so that treatments such as neural mobilization can be directed to those patients most likely to benefit. Further studies will be required to determine the effectiveness of neural mobilization for patients with PNS.
Despite the simplicity of the classification system, to ensure that it was uniformly executed in the study, all examining therapists participated in pre-study training by Th and AS and were provided with a procedure instruction booklet for reference. Veracity of the data was maintained by strict blinding of the examiners. Other strengths of the study include complete reporting of frequencies of outcome and agreement (Table 3) and data analysis implementing kappa statistics and 95% CIs.
A limitation of our study is that pairing of examiners and the order of testing were not randomized. Instead, the first author was always part of the examiner pair, and order of testing was dictated by organizational circumstances. This limitation may be countered by the fact that this examination process reflects a more typical clinical situation as encountered in everyday practice.
The reliability of a number of examination procedures and five classification systems for patients with nonspecific LBP has been investigated. These studies have recently been reviewed47, and it was found that results of the five examined LBP classification systems13–15,48,49 were often conflicting and that interpretation was confounded by variations in the quality and design of the studies. For example, one important point in regards to study design is the procedure of examination. Assessments may be carried out simultaneously or consecutively. In general, for a simultaneous assessment design, both examiners are present during the examination. Alternately, photos, videotapes, and documented subjective examination may be used in place of attendance at the clinical examination. While simultaneous assessments have the advantage of eliminating variations of patients' reports and behavior between two sessions and the effects of repeated testing, which may alter symptom response, these may also introduce bias and thereby artificially inflate the k value. In contrast, consecutive assessments may be confounded by real changes in the condition of the patients, either secondary to or independent of the initial examination. The interval between consecutive examinations must be individualized to the investigated pathology in the study group and the nature of the examination.
Three of the classification systems reviewed by May et al47 were relevant in regards to radiating leg pain. The McKenzie classification system13 was most extensively investigated with four studies providing conflicting evidence on interrater reliability with kappa values ranging from 0.26 to 1.0050–53.
The patho-anatomic-based diagnostic classification system15 has shown fair to excellent agreement on syndromes reported by one high-quality study, with kappa values ranging from 0.44 to 1.00 for diagnosis of syndromes but poor reliability for the classification system as a whole (overall agreement on all syndromes for each patient, as syndromes can coexist) with 39% agreement54. The treatment-based classification system14 demonstrated fair to good agreement55,56 for the overall classification system.
When comparing results of reliability studies, other factors also have to be taken into account. Although the k coefficient is widely acknowledged as the appropriate statistic for analyzing categorical data, there are some issues that may confound the interpretation of k values. The k coefficient indicates the proportion of achieved agreement between examiners beyond agreement by chance alone46. Two factors influence the magnitude of the k coefficient: the prevalence of a symptom or syndrome and the pattern of disagreement (bias) between observers57. Feinstein and Cicchetti57 found two paradoxes in regards to prevalence and bias. The first paradox is the case of either the high or low prevalence of a symptom or syndrome, where agreement per chance would be high and the k coefficient would be accordingly reduced. A good example for this paradox is found in a study measuring interrater reliability of the McKenzie classification system where both examiners assigned the patients mainly into the derangement syndrome group (90%; 35 of 39 patients)53. Although percentage agreement was high at 95%, the k coefficient was relatively low at 0.60. In our study, the proportion of subjects in the CS, D, and M groups were reasonably evenly distributed (32.5%, 30%, and 27.5%, respectively), whereas prevalence of subjects in the PNS group was low at only 10%. The low prevalence of PNS may have deflated the k value in our study. The extent to which low prevalence in a study sample is a mirror of the true prevalence in the population or whether it rather reflects the clinicians' diagnostic behavior is questionable58. In addition to prevalence, it is also possible that bias may have influenced k values in our study, but this could not be tested as calculating bias is only suitable for 2×2 tables58.
Also the number of diagnostic categories should be taken into account when comparing k statistics for classification systems. The larger the number of categories, the more difficult it is for observers to agree. Additionally, a higher number of examiners will make agreement more complex while improving the quality of a study. Therefore, multiple pairs of examiners are recommended47,59.
When interpreting k coefficients as outcomes of reliability research, one also has to consider the condition/pathology for which the investigated test is used. In the case of diagnosing serious pathology, interrater agreement for diagnostic tests should be as good as possible; in this case, even a k score of above 0.90 may not be satisfactory, as the consequences of a misdiagnosis may be fatal. On the other hand, most measurements in musculoskeletal medicine do not investigate life-threatening conditions; therefore, lower k values may be considered satisfactory.
Validation of a classification system is a multi-step process41. We have established that the classification system we propose is reliable when used by experienced physiotherapists. The next step is to investigate criterion-related validity of the classification system. Once this has been established, studies of the effectiveness of selected treatment techniques can be conducted in more homogenous mechanism-based subgroups of patients.
Conclusion
There was good inter-examiner agreement for the mechanism-based classification system for low back-related leg pain. Therefore, further investigation of the validity of the classification is justified.
Acknowledgements
The authors would like to thank Dr. Gerd Müller, Kerstin Brass, Lucia Grauel, Frank Kammeier, and Kirsten Keller of the Rückenzentrum am Michel, hamburg, for their support and assistance in this investigation. In addition, the authors would like to thank Dr. Rebolledo, Dr. Poetsch, and Dr. Thomsen for their support in patient recruitment and Dr. Ritu Gupta for statistical support.
Appendix A: Physical Examination Procedur
Central Sensitization
The subjective examination incorporates the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) scale, a screening tool for neuropathic symptoms and signs indicative of central processes enhancing sensitivity of the sensory system. Subjects with a score of 12 points or above (of 24) on the LANSS scale were classified in the group Central Sensitization.
Denervation
The physical examination included neurological examination, assessment of active movements, and neural tissue provocation tests. A neurological examination was carried out that included assessment of reflexes, muscular strength, and altered sensitivity for pinprick (Ad) and light touch (Ab fibers). These tests were screening for negative signs and were therefore considered positive in the presence of diminished or absent reflexes, muscle power, or sensation on the symptomatic side. Subjects were classified in the Denervation group if they had at least two or more positive tests in two different categories (i.e., reflexes, muscle power, light touch, or pinprick sensitivity).
Peripheral Nerve Sensitization
Following the neurological examination was an investigation of signs indicative of peripheral sensitization of the nerve trunk with enhanced mechano-sensitivity. These signs included restricted range of active movement in standing and hyperalgesia on neural tissue provocation tests (e.g., slump test or SLR, and nerve trunk palpation), both of which had to correlate with the suspected nerve trunk that was mechano-sensitive. Positive neural tissue provocation tests, in the absence of positive symptoms and neurological deficit, were classified as indicative of peripheral sensitization of the nerve trunk with enhanced mechanosensitivity. The flexion active range of motion test (AROMF) was carried out in standing, with the patient's feet slightly apart. The patient was asked to bend forward, while keeping the knees straight, to the first onset of discomfort. Finger tip to floor distance was measured and in case of reproduction of leg pain, the test was repeated with a wedge under the foot of the symptomatic leg to increase ankle dorsiflexion. The AROMF test was considered positive if with increased ankle dorsiflexion reproduction of leg pain was more severe.
The SLR test was carried out in supine lying, with a bubble goniometer (MIE, Leeds, UK) held on the tibial tubercle to measure SLR range of motion. The movement of SLR was carried out to the first onset of discomfort and range of motion recorded. If the patient's leg pain was provoked, then the test was repeated with the ankle held in dorsiflexion. The SLR test was only considered positive if the patient's leg pain was more severe when the SLR test was repeated with ankle dorsiflexion.
The prone knee bend (PKB) test was conducted in prone lying, with the goniometer held in the middle of the tibia to measure knee range of motion. The knee was flexed until the first onset of discomfort. If the patient's leg pain was reproduced, the test was repeated with the patient's spine laterally flexed to the contralateral side. The PKB test was only considered positive if the patient's leg pain was more severe when the PKB test was repeated with the spine in contralateral lateral flexion.
Lower limb peripheral nerve trunks were palpated at the following sites: the sciatic nerve immediately lateral to the ischial tuberosity, the tibial nerve in the mid-line of the popliteal fossa, the common peroneal nerve behind the head of the fibula, and the femoral nerve immediately lateral to the femoral artery level with the inguinal ligament. Nerve palpation tests were considered positive if palpation was more painful on the symptomatic leg compared to the asymptomatic side. All tests were carried out first on the asymptomatic and then on the symptomatic leg. All components had to be positive for Peripheral Nerve Sensitization to be present. The procedure and rationale for this examination protocol are described in more detail elsewhere60.
AROMF, SLR, or PKB and nerve palpation all had to be positive for the patient to be classified as PNS.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
REFERENCES
- 1.Borkan JM, Koes B, Reis S, Cherkin DC. A report from the Second International Forum for Primary Care Research on Low Back Pain: Reexamining priorities. Spine. 1998;23:1992–1996. doi: 10.1097/00007632-199809150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Henschke N, Maher CG, Refshauge KM, Das A, McAuley JH. Low back pain research priorities: A survey of primary care practitioners. BMC Fam Prac. 2007;8:40. doi: 10.1186/1471-2296-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Boxem K, Van Zundert J, Van Zundert J, Patijn J, van Kleef M. Pseudoradicular and radicular low-back pain: How to diagnose clinically? Pain. 2008;135:311–312. doi: 10.1016/j.pain.2008.02.003. author reply 313–315. [DOI] [PubMed] [Google Scholar]
- 4.Leffler AS, Hansson P. Letter to the editor of Pain on Freynhagen et al: Pseudoradicular and radicular low-back pain: A disease continuum rather than different entities? Answers from quantitative sensory testing. Pain. 2007;135:65–74. doi: 10.1016/j.pain.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh JM, Weinstein JN. Low back pain: Epidemiology, anatomy and neurophysiology. In: Wall PD, Melzack R, editors. The Textbook of Pain. New York: Churchill Livingstone; 1994. [Google Scholar]
- 6.Heliövaara M, Impivaara O, Sievers K. Lumbar disc syndrome in Finland. J Epid Com Health. 1987;41:251–258. doi: 10.1136/jech.41.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selim AJ, Ren XS, Fincke G, et al. The importance of radiating leg pain in assessing health outcomes among patients with low back pain: Results from the Veterans Health Study. Spine. 1998;23:470–474. doi: 10.1097/00007632-199802150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Freynhagen R, Baron R, Gockel U, Tölle TR. Screening of neuropathic pain components in patients with chronic back pain associated with nerve compression: A prospective observational study (MIPORT) Curr Med Res Op. 2006;22:529–537. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 9.Merskey H, Bogduk N. Classi"cation of Chronic Pain. 2nd ed. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 10.Bogduk N, McGuirk B. Medical Management of Acute and Chronic Low Back Pain: An Evidence-Based Approach. Amsterdam, the Netherlands: Elsevier; 2002. [Google Scholar]
- 11.Vroomen PC, de Krom MC, Slofstra PD, Knottnerus JA. Conservative treatment of sciatica: A systematic review. J Spinal Disord. 2000;13:463–469. doi: 10.1097/00002517-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Luijsterburg PA, Verhagen AP, Ostelo RW, van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: A systematic review. Eur Spine J. 2007;16:881–899. doi: 10.1007/s00586-007-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenzie RA. The Lumbar Spine: Mechanical Diagnosis and Therapy. Waikanae, NZ: Spinal Publications; 1981. [Google Scholar]
- 14.Delitto A, Erhard RE, Bowling RW. A treatment-based classification approach to low back syndrome: Identifying and staging patients for conservative treatment. Phys Ther. 1995;75:470–485. doi: 10.1093/ptj/75.6.470. discussion 485–479. [DOI] [PubMed] [Google Scholar]
- 15.Petersen T, Laslett M, Thorsen H, Manniche C, Ekdahl C, Jacobson S. Diagnostic classification of non-specific low back pain: A new system integrating pathoanatomic and clinical categories. Physiother Theory Pract. 2003;19:213–237. [Google Scholar]
- 16.Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593–2602. doi: 10.1097/01.brs.0000146464.23007.2a. [DOI] [PubMed] [Google Scholar]
- 17.Fritz JM, Lindsay W, Matheson JW, et al. Is there a subgroup of patients with low back pain likely to benefit from mechanical traction? Results of a randomized clinical trial and subgrouping analysis. Spine. 2007;32:E793–E800. doi: 10.1097/BRS.0b013e31815d001a. [DOI] [PubMed] [Google Scholar]
- 18.Browder DA, Childs JD, Cleland JA, Fritz JM. Effectiveness of an extension-oriented treatment approach in a subgroup of subjects with low back pain: A randomized clinical trial. Phys Ther. 2007;87:1608–1618. doi: 10.2522/ptj.20060297. discussion 1577–1609. [DOI] [PubMed] [Google Scholar]
- 19.Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: Results of a randomized clinical trial. Spine. 2006;31:623–631. doi: 10.1097/01.brs.0000202807.72292.a8. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol. 2006;96:579–590. doi: 10.1152/jn.00087.2006. [DOI] [PubMed] [Google Scholar]
- 22.Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–1955. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- 23.Eliav E, Benoliel R, Tal M. Inflammation with no axonal damage of the rat saphenous nerve trunk induces ectopic discharge and mechanosensitivity in myelinated axons. Neuroscience Letters. 2001;311:49–52. doi: 10.1016/s0304-3940(01)02143-7. [DOI] [PubMed] [Google Scholar]
- 24.Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:169–182. doi: 10.1016/s0304-3959(99)00102-5. [DOI] [PubMed] [Google Scholar]
- 25.Woolf CJ, Mannion RJ. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: Implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer A, Hall T, Briffa K. Classification of low back-related leg pain: A proposed patho-mechanism-based approach. Man Ther. 2009;14:222–230. doi: 10.1016/j.math.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Elvey RL, Hall TM. Neural tissue evaluation and treatment. In: Donatelli R, editor. Physical Therapy of the Shoulder. New York: Churchill Livingstone; 1997. [Google Scholar]
- 29.Elvey R. Treatment of arm pain associated with abnormal brachial plexus tension. Austr J Physiother. 1986;32:225–230. doi: 10.1016/S0004-9514(14)60655-3. [DOI] [PubMed] [Google Scholar]
- 30.Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervicobrachial pain syndrome: A pilot study. Man Ther. 2002;7:95–102. doi: 10.1054/math.2002.0453. [DOI] [PubMed] [Google Scholar]
- 31.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J Orthop Sports Phys Ther. 2003;33:369–378. doi: 10.2519/jospt.2003.33.7.369. [DOI] [PubMed] [Google Scholar]
- 32.Cowell IM, Phillips DR. Effectiveness of manipulative physiotherapy for the treatment of a neurogenic cervicobrachial pain syndrome: A single case study–experimental design. Man Ther. 2002;7:31–38. doi: 10.1054/math.2001.0429. [DOI] [PubMed] [Google Scholar]
- 33.Hall T, Elvey R, Davies N, Dutton L, Moog M. Efficacy of manipulative physiotherapy for the treatment of cervicobrachial pain. 73–74. Presentation at 10th Manipulative Physiotherapists Association of Australia Conference. Melbourne, Australia, 1997.
- 34.Cleland JA, Hunt GC, Palmer JA. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: A single case design. J Man Manip Ther. 2004;12:143–152. [Google Scholar]
- 35.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: A pilot clinical trial. Man Ther. 2006;11:279–286. doi: 10.1016/j.math.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Cibulka MT, Aslin K. How to use evidencebased practice to distinguish between three different patients with low back pain. J Orth Sports Phys Ther. 2001;31:678–688. doi: 10.2519/jospt.2001.31.12.678. discussion 689–695. [DOI] [PubMed] [Google Scholar]
- 37.Kitteringham C. The effect of straight leg raise exercises a"er decompression surgery: A pilot study. Physiother. 1996;82:115–123. [Google Scholar]
- 38.Jull G, Sterling M, Kenardy J, Beller E. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash? A preliminary RCT. Pain. 2007;129:28–34. doi: 10.1016/j.pain.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Lidbeck J. Central hyperexcitability in chronic musculoskeletal pain: A conceptual breakthrough with multiple clinical implications. Pain Res Man. 2002;7:81–92. doi: 10.1155/2002/310974. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain. Pain. 1998;77:227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 41.Ford J, Story I, O'Sullivan P, McMeeken J. Classification systems for low back pain: A review of the methodology for development and validation. Phys Ther Rev. 2007;12:33–42. [Google Scholar]
- 42.Bennett M. The LANSS pain scale: The Leeds Assessment of Neuropathic Symptoms and Signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 43.Hall TM, Elvey RL. Nerve trunk pain: Physical diagnosis and treatment. Man Ther. 1999;4:63–73. doi: 10.1054/math.1999.0172. [DOI] [PubMed] [Google Scholar]
- 44.Byrt T. How good is that agreement? Epidemiology. 1996;7:561. doi: 10.1097/00001648-199609000-00030. [DOI] [PubMed] [Google Scholar]
- 45.Fleiss JL, Levin BA, Paik MC. Statistical Methods for Rates and Proportions. 3rd ed. Wiley Series in Probability and Statistics. Hoboken, NJ: John Wiley & Sons; 2003. [Google Scholar]
- 46.Landis JR, Koch GG. The measurement for observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 47.May S, Littlewood C, Bishop A. Reliability of procedures used in the physical examination of non-specific low back pain: A systematic review. Austr J Physiother. 2006;52:91–102. doi: 10.1016/s0004-9514(06)70044-7. [DOI] [PubMed] [Google Scholar]
- 48.Sahrmann S. Diagnosis and Treatment of Movement Impairment Syndromes. St. Louis, MO: Mosby; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall H, McIntosh G, Melles T. A different approach to back pain diagnosis: Identifying a pattern of pain. Can J Cont Med Ed. 1994;6:31–42. [Google Scholar]
- 50.Razmjou H, Kramer JF, Yamada R. Intertester reliability of the McKenzie evaluation in assessing patients with mechanical lowback pain. J Orthop Sports Phys Ther. 2000;30:368–383. doi: 10.2519/jospt.2000.30.7.368. discussion 384–389. [DOI] [PubMed] [Google Scholar]
- 51.Clare HA, Adams R, Maher CG. Reliability of McKenzie classification of patients with cervical or lumbar pain. J Manipulative Physiol Ther. 2005;28:122–127. doi: 10.1016/j.jmpt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Riddle DL, Rothstein JM. Intertester reliability of McKenzie's classifications of the syndrome types present in patients with low back pain. Spine. 1993;18:1333–1344. doi: 10.1097/00007632-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Kilpikoski S, Airaksinen O, Kankaanpaa M, Leminen P, Videman T, Alen M. Interexaminer reliability of low back pain assessment using the McKenzie method. Spine. 2002;27:E207–E214. doi: 10.1097/00007632-200204150-00016. [DOI] [PubMed] [Google Scholar]
- 54.Petersen T, Olsen S, Laslett M, et al. Intertester reliability of a new diagnostic classification system for patients with non-specific low back pain. Austr J Physiother. 2004;50:85–94. doi: 10.1016/s0004-9514(14)60100-8. [DOI] [PubMed] [Google Scholar]
- 55.Fritz JM, Brennan GP, Clifford SN, Hunter SJ, Thackeray A. An examination of the reliability of a classification algorithm for subgrouping patients with low back pain. Spine. 2006;31:77–82. doi: 10.1097/01.brs.0000193898.14803.8a. [DOI] [PubMed] [Google Scholar]
- 56.Fritz JM, George S. The use of a classification approach to identify subgroups of patients with acute low back pain: Interrater reliability and short-term treatment outcomes. Spine. 2000;25:106–114. doi: 10.1097/00007632-200001010-00018. [DOI] [PubMed] [Google Scholar]
- 57.Feinstein AR, Cicchetti DV. High agreement but low kappa. I. The problems of two paradoxes. J Clin Epid. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- 58.Sim J, Wright CC. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–268. [PubMed] [Google Scholar]
- 59.van der Wurff P, Hagmeijer RH, Meyne W. Clinical tests of the sacroiliac joint: A systemic methodological review. Part 1: Reliability. Man Ther. 2000;5:30–36. doi: 10.1054/math.1999.0228. [DOI] [PubMed] [Google Scholar]
- 60.Hall TM, Elvey RL. Management of mechanosensitivity of the nervous system in spinal pain syndromes. In: Boyling JD, Jull G, editors. Grieves Modern Manual Therapy. Edinburgh, UK: Churchill Livingstone; 2004. [Google Scholar]