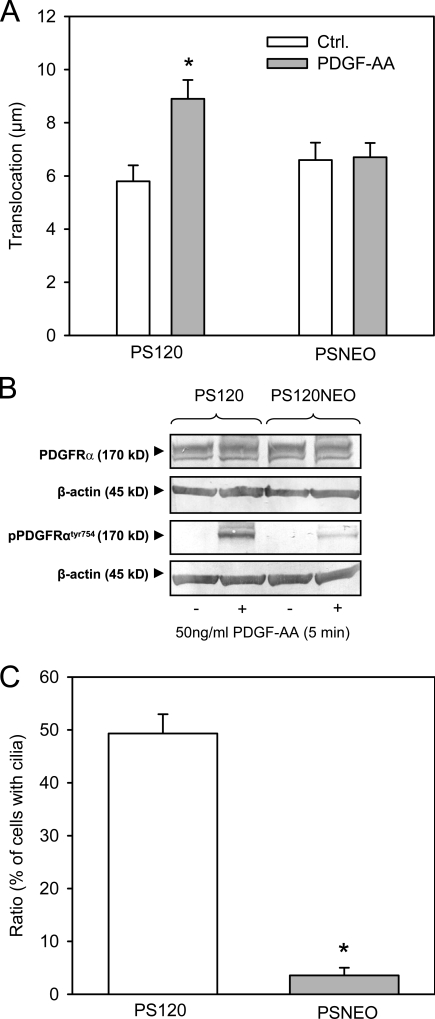
Figure 7.
Migration, PDGFR-α activation, and cilia formation in NHE1-null and NHE1-expressing cells. (A) PS120 (expressing NHE1) and PSNEO (deficient for NHE1) cells were serum starved for 48 h to induce growth arrest, and migration was analyzed in 4-h wound-healing assays in the absence and presence of 50 ng/ml PDGF-AA, as indicated. The graph shows the mean translocation ± SEM of 45–64 cells in four to six independent experiments. Ctrl., control. (B) PS120 and PSNEO (PS120NEO) cells were serum starved for 48 h, exposed to 50 ng/ml PDGF-AA, and lysed, and Western blots of total and Tyr754-phosphorylated PDGFR-α were performed as described in Materials and methods. β-Actin was used as a loading control. As seen, PDGFR-α expression was similar in the two cell types, whereas PDGF-AA–induced phosphorylation of PDGFR-α was reduced, although not abolished, in the PSNEO cells. Data shown are representative of three independent experiments for each cell line. (C) PS120 and PSNEO cells were serum starved for 48 h to induce growth arrest, paraformaldehyde fixed, and labeled for acetylated α-tubulin to visualize primary cilia. Approximately 300 randomly chosen cells from each experiment were analyzed for the presence or absence of primary cilia, and the results shown are the mean ± SEM of the percentage of cells with cilia in six independent sets of paired experiments. (A and C) Data were analyzed using parametric or nonparametric ANOVA, and the level of significance is shown (*, P < 0.05).