Figure 4.
SCaBP8 Phosphorylation Takes Place at the Membrane.
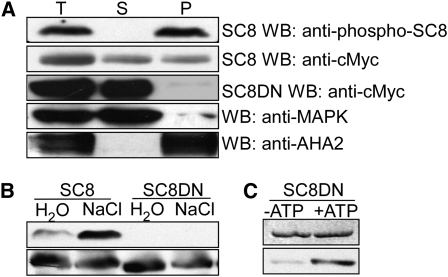
(A) SCaBP8Ser237 phosphorylation was detected only in membrane fractions. Seedlings of wild-type (Col-0) plants expressing 6myc-SCaBP8 or 6myc-SCaBP8DN, in which a 25–amino acid hydrophobic domain in the N terminus of SCaBP8 that is required for SCaBP8 plasma membrane localization was removed, were treated with 100 mM NaCl for 3 h. Membrane and soluble fractions were separated by centrifugation at 4°C for 60 min at 140,000g. Equal aliquots of total (T), supernatant (S), and pellet (P) proteins were separated by SDS-PAGE followed by analysis with anti-myc (loading control), anti-phosphoserine237, anti-MAPK3 (loading control for a soluble-protein fraction), or anti-AHA2 antibodies (loading control for a cell membrane fraction).
(B) SCaBP8Ser237 phosphorylation was not detected in 6myc-SCaBP8DN transgenic plants. Seedlings of wild-type (Col-0) plants expressing 6myc-SCaBP8 or 6myc-SCaBP8DN were treated with 100 mM NaCl or water for 3 h. Proteins were extracted and analyzed with anti-phosphoserine237 (top panel) or anti-myc (bottom panel) antibodies.
(C) Anti-phosphoserine237 antibodies detect SCaBP8Ser237 phosphorylation of SCaBP8DN in vitro. SDS-PAGE with Coomassie blue–stained SCaBP8 proteins (top panel); autoradiograph showing SCaBP8DN phosphorylation detected by anti-phosphoserine237 antibodies (bottom panel).
SC8DN, SCaBP8DN in which a 25–amino acid hydrophobic domain in the N terminus has been deleted; AHA2, isoform of the plasma membrane H+-ATPAse; MAPK3, mitogen-activated protein kinase3.