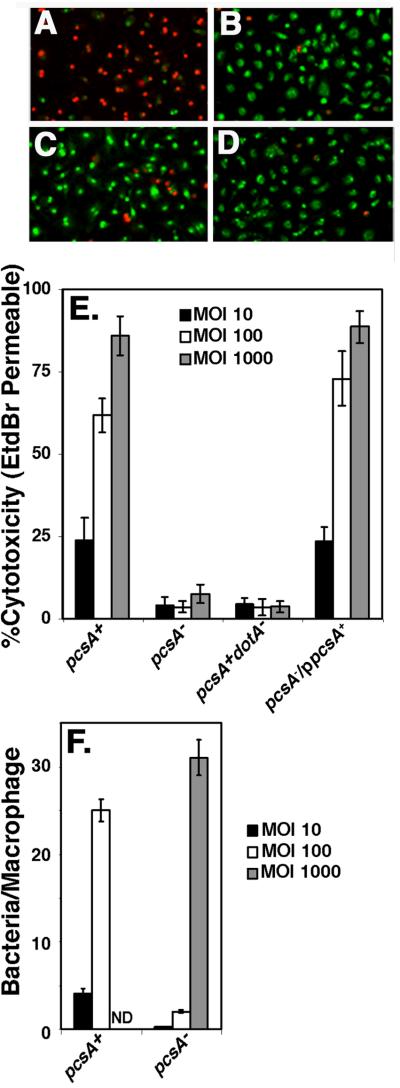
Figure 5. The defect in Dot/Icm-dependent cytotoxicity caused by the absence of PC is not the result of lack of adhesiveness.
Bacteria were allowed to incubate with macrophages for 55 min at 37°C at the indicated series of MOI with the following strains: pcsA+, GL84; pcsA−, GL263; pcsA+dotA−, GL83; pcsA−/ppcsA+, GL266. Merged images of 10X magnifications of incubations with bone marrow macrophages incubated at an MOI = 100 are displayed for: (A) dotA+pcsA+, (B) dotA− pcsA+, (C) pcsA− dotA+, and (D) lidC dotA+ (Conover et al., 2003). Green cells are those staining with acridine orange, while red cells are those that are dead and permeable to EtdBr. (E). Defective cytotoxicity in a pcsA− mutant is independent of bacterial dose. Cytotoxicity was measured by identifying cells permeable to ethidium bromide (EtdBr), which was added after removing the medium immediately after the infection period. % cytotoxicity was determined as the percentage of cells observed to be EtdBr-permeable in the rhodamine fluorescence channel relative to the total number of acridine orange stained cells observed in the FITC channel, using grabbed images (Experimental Procedures). (F) Incubation of the pcsA− mutant at high multiplicity allows adhesion of bacteria to macrophages. In parallel to the cytotoxicity assay, incubations were fixed, bacteria were identified with an anti-L. pneumophil antibody and the number of bacteria bound per macrophage was determined at the noted MOI. Strains are same as in panel (E). Quantitation of binding at MOI = 1000 for the pcsA+ strain was not determined (ND). All experiments were repeated twice.