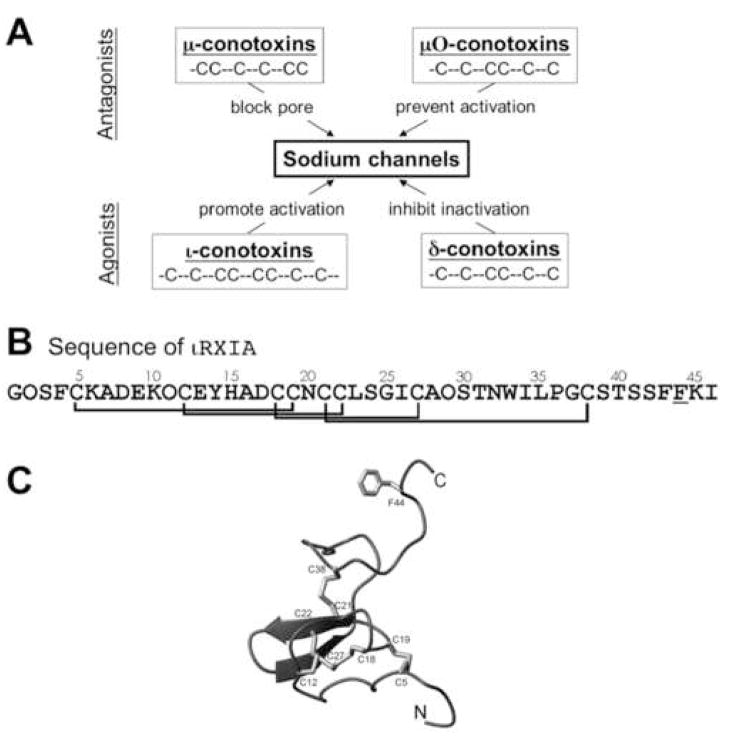
Figure 1.
A, Four families of peptide conotoxins that target Na channels. Members in the upper two, μ- and μO-conotoxins, inhibit Na channels, whereas those in the lower two, ι- and δ-conotoxins, are agonist. The cysteine framework of each peptide family is illustrated. All cysteines are disulfide-bonded, rendering each peptide into a compact structure. B, The sequence of ι-RXIA, the iota-conotoxin studied in this report, where O is hydroxyproline, and the underlined F (residue 44) is the D-enantiomer of phenylalanine. The synthetic homologue, ι-RXIA[L-Phe44], is identical to ι-RXIA except residue 44 is an L-phenylalanine. The disulfide bridges between Cys residues are indicated. C, Ribbon structure of ι-RXIA determined by NMR spectroscopy [20]. Locations of the Cys residues and their disulfide linkages are indicated; also shown is the side chain of D-Phe44. This figure was prepared using PyMol (http://www.pymol.org).