Fig. 2.
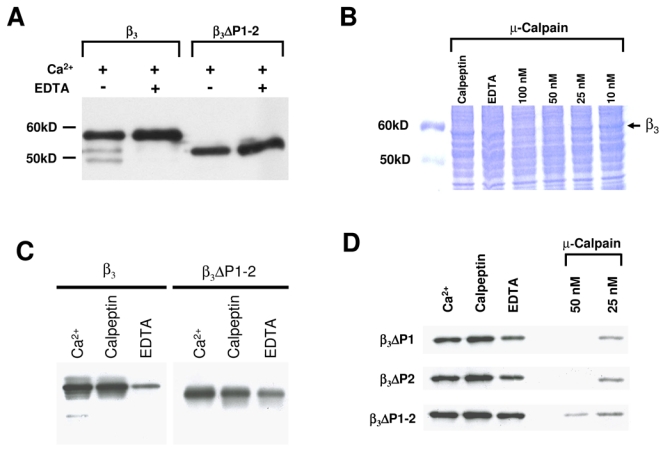
The CaVβ3 subunit is cleaved by Ca2+-dependent proteases. A, Recombinant CaVβ3 ΔP1–2 protein is more stable than the wild-type CaVβ3 to endogenous Ca2+-dependent proteases. Lanes 1 and 2 show the proteolytic breakdown of wild-type CaVβ3 (β3) heterologously expressed in HEK- 293 cells. 200 μg of microsomes were incubated with Ca2+ (750 μM) at 30°C for 20 min in absence or presence of EDTA (1.5 mM). No proteolytic degradation was observed (lanes 3–4) when the PEST-like regions were deleted (ΔP1–2). B, Coomassie-stained SDS-PAGE in a 10% resolving gel using Laemmli buffer system. Lane 1, control; lanes 2–7, microsomes from HEK- 293 cells expressing the wild-type CaVβ3 incubated for 20 min at 30°C with Ca2+, EDTA or calpeptin or exposed to increasing concentrations of μ-calpain. C, Degradation of the CaVβ3 subunits in the presence of Ca2+ and a calpain protease inhibitor. CaVβ3 subunits were incubated at 30°C for 20 min with 750 μM Ca2+, 180 nM of calpeptin or 1.5 mM EDTA. The full-length (β3) or the double PEST deletion mutant (ΔP1–2) are indicated. D, ΔPEST mutant CaVβ3 proteins were incubated for 20 min at 30°C with Ca2+, EDTA or calpeptin as indicated above or exposed to two increasing doses of μ-calpain as listed (in the presence of 750 μM Ca2+). In each case, one representative of at least two independent degradation experiments of wild-type and mutant CaVβ3 proteins is presented.
