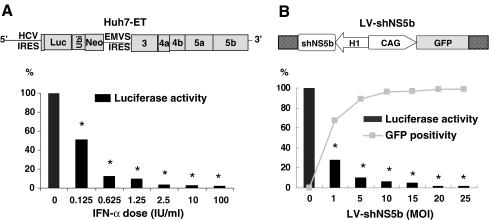
Fig. 1.
Inhibition of HCV replication by IFN-α or LV-shNS5B. a Huh7-ET replicon was used for testing HCV replication by monitoring luciferase activity. IFN-α treatment inhibits viral replication in a dose-dependent manner. Profound reduction of luciferase activity (97 ± 2% inhibition, mean ± SD, n = 9) was observed from 2.5 to 100 IU/ml concentration of IFN-α. b LV-shNS5b contains both GFP reporter gene and shRNA targeting HCV were tested. Huh7-ET treated with increasing dose of LV-shNS5b resulted in higher levels of transduction efficiency and inhibition of HCV replication, monitored by GFP-positive population and luciferase activity, respectively. Maximum inhibition of HCV replication was observed at high dose (20 or 25 MOI) by 98 ± 3% (mean ± SD, n = 6). *P < 0.01 (Wilcoxon test) significantly different from untreated conditions