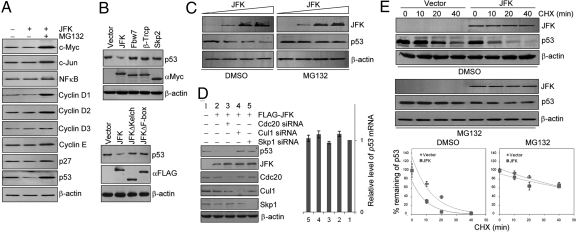
Fig. 2.
JFK promotes p53 turnover via an SCF-dependent mechanism. (A) JFK destabilizes p53 protein. U2OS cells were transfected with JFK. Cellular extracts were prepared under no treatment or treatment with MG132 and analyzed by Western blotting for protein expression with antibodies against the indicated proteins. (B) (Upper) p53 degradation is specific to JFK. U2OS cells were transfected with Myc-tagged JFK, Fbw7, β-TrCP, or Skp2. Cellular extracts were prepared and Western blotting was performed with anti-p53. (Lower) WT JFK but not JFK mutants promote p53 degradation. U2OS cells were transfected with FLAG-JFK, FLAG-JFKΔKelch, or FLAG-JFKΔF-box. Cellular extracts were prepared and Western blotting was performed with anti-p53. (C) JFK decreases the steady-state level of exogenous p53 protein. H1299 cells were co-transfected with a constant amount of p53 and increasing amounts of FLAG-JFK. Forty-eight hours after transfection, the cells were treated with vehicle (DMSO) or MG132 for 6 h before cells were collected for Western blotting analysis. (D) (Left) The destruction of p53 by JFK is mediated by an SCF complex. U2OS cells were transfected with JFK together with siRNAs for knocking down Cul1, Skp1, or Cdc20 as indicated. Cellular proteins were extracted for Western blotting analysis with antibodies against the indicated proteins. (Right) JFK does not alter p53 mRNA levels. Total RNAs were extracted from the above-described U2OS cells and analyzed for p53 expression by real-time RT-PCR. The data were normalized to GAPDH. Each bar represents the mean ± SD for triplicate experiments. (E) JFK decreases p53 half-life. U2OS cells were transfected with vector or FLAG-JFK. Forty-eight hours after transfection, cells were treated with vehicle (DMSO) or MG132 for 6 h before addition of cycloheximide (CHX; 50 μg/mL). Cells were then harvested for Western blotting analysis at the designated times after CHX treatment. Quantitation was done by densitometry and expressed as signals of p53/β-actin. Each point represents the mean ± SD for triplicate experiments.