Abstract
Compound adhesives made from red ochre mixed with plant gum were used in the Middle Stone Age (MSA), South Africa. Replications reported here suggest that early artisans did not merely color their glues red; they deliberately effected physical transformations involving chemical changes from acidic to less acidic pH, dehydration of the adhesive near wood fires, and changes to mechanical workability and electrostatic forces. Some of the steps required for making compound adhesive seem impossible without multitasking and abstract thought. This ability suggests overlap between the cognitive abilities of modern people and people in the MSA. Our multidisciplinary analysis provides a new way to recognize complex cognition in the MSA without necessarily invoking the concept of symbolism.
Keywords: ochre; replications; glue; modern behavior; >70,000 years ago
Archaeologists often use symbolic material culture as a marker of modern behavior, but few agree on definitions of either term or explore the types of mental architecture required for symbolic innovations. Here, we move away from the contentious issue of symbolism and draw on the combined expertise of cognitive and earth scientists to create a fresh way of recognizing, in the deep past, cognitive abilities that overlap with our own. People today have a capacity for novel, sustained multilevel operations; this ability may have arisen from neural connectivity in part of the prefrontal cortex (1). The capacity may be recognizable in some technologies, and we use compound adhesive manufacture as our example. To demonstrate complex cognition, we must show that some executive steps required for compound adhesive manufacture are not possible without mental abilities of the kind implied in the ninth subsystem of the Barnard et al. (2) model of mental architecture. Here, abstract meanings and sophisticated organization of action sequences determine decision making. An earlier eighth subsystem would have been mentally incapable of processing 2 levels of meaning simultaneously or of generating fully abstract concepts about behavior.
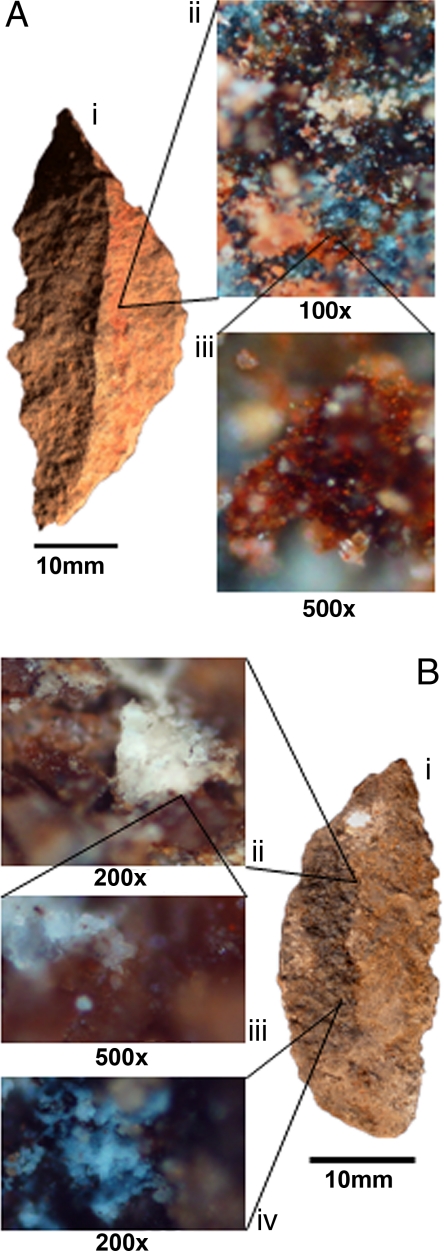
The use of simple (1-component) adhesives is ancient; for example, birch-bark tar was found on 2 flakes from ≈200,000 years (200 ka) ago at a site in Italy (3). At ≈40 ka, bitumen was found on stone tools in Syria (4), and a similarly aged site in Kenya yielded tools with red ochre stains that imply the use of multicomponent glue (5). Traces of even earlier (≈70 ka) compound adhesives occur, together with microfractures consistent with hafting, on Middle Stone Age (MSA) stone tools from Sibudu Cave, South Africa (see SI Text and Table S1). Several recipes are evident: sometimes plant gum and red ochre (natural iron oxide–hematite–Fe2O3) traces (Fig. 1) occur on tool portions that were once inserted in hafts (6–10). Other tools have brown plant gums and black or white fat, but no ochre (Fig. 1 and SI Text).
Fig. 1.
Stone tools (segments) with adhesive from Sibudu Cave. (A) Segment with red ochre visible to the naked eye on that part of the tool that would have been in a haft (i). Microscopic views of red ochre and plant gum on the tool (ii and iii). (B) Segment with black fat on that part of the tool that would have been in a haft (i). Microscopic views of fat on the tool (ii–iv).
We acknowledge the possibility that people at Sibudu colored some of their weapons symbolically, perhaps to signify the blood of prey, but we do not find the explanation entirely satisfying. First, when red ochre stains are present, they are only on those parts of stone tools once attached to hafts (Fig. 1); weapons were not anointed with ochre, as occurred historically (11). Second, not all MSA adhesives contain red ochre, as might be expected if color was considered important to the success of the weapon. Plant gum used alone sticks effectively, but often shatters on impact, a characteristic favored only when weapon tips are intended to break inside prey (12). Adding ochre to gum seems to create a less brittle product and act as a desiccant to prevent gum from dissolving under damp conditions (13). Thus, we suspect that artisans selected task-appropriate glue recipes. Third, although ochre traces found on tools are predominantly red, hematite may not always have been the original ingredient and may be archaeologically overrepresented. Goethite (yellow ochre, the oxide–hydroxide α-FeOOH) could equally have been used because there are several pathways from this product to hematite (14). If goethite is heated to ≈350 °C, it dehydroxylates, with corresponding color change from yellow to red (14, 15). Color transformation through heating could occasionally have been accidental because postdepositional heating affects buried items (16, 17), including yellow ochre (18). Because many artifacts become accidentally buried under or near ancient camp fires, which are abundant at sites like Sibudu, it should be expected that some yellow nodules and adhesives reddened postdepositionally. This hypothesis can be tested in the future because crystal structures of heated and unheated ochre differ (19).
Why might plant gum and ochre be combined satisfactorily to create compound adhesive? An analogy from nature provides the first clue: Acacia gum exudes from wounds to seal the tree. This gum is also used in the food industry as an emulsifier and stabilizer of suspensions (20). Iron is the fourth most abundant element (14), and in nature, free iron or goethite becomes water-soluble and mobile when it combines chemically with acidophyle plants (21). Indeed, goethite's solubility increases by 6 orders of magnitude when organic acids are added (22). Because Acacia karroo gum has an uronic acid component (23), it is ideally suited for the chemical complexing of iron, which may have taken place when compound glues were made.
To explore first the effect of using ochre as a loading agent in plant gum glues and, second, the complexity of thought processes involved in compound adhesive manufacture, we replicated hafted tools by using only products and methods that would have been available in the MSA. The choice of natural products means that our methodology differs from laboratory-based experiments in which iron oxides and oxyhydroxides are synthetically produced with controlled pH, ionic strength, and temperature. Natural products and wood fires incorporate variables that are disastrous by laboratory standards, but ancient artisans were obliged to deal with these. For example, the viscosity and pH of Acacia gum from individual trees fluctuates, and ochre samples from both the same and geographically distant outcrops can have markedly different mineralogy (24) (Table S2). Fe2O3 content of ochre varies: readings of 48–91% were obtained for archaeologically recovered red ochre nodules from Sibudu and Rose Cottage (Table S2). Yellow ochres from geological sources near these sites contain between 15% and 25% Fe, yet Thabazimbi goethite has high iron content (Table S2). Such variables imply that ancient adhesives may not have been made by using standard recipes, but that on-the-spot adaptations, requiring cognitive fluidity, were necessary.
Replication Results
The consistently most successful adhesives—that is, those that did not allow the stone insert to break from its haft when the tool was used—were those with red ochre no. 15 (95% success rate; Table 1). Adhesives incorporating small amounts of beeswax were always successful. Adhesives with red synthetic hematite were always unsuccessful. Success rates varied for adhesives containing the powder from other iron oxides or oxyhydroxides (Table 1), so clearly not all ochre is suitable as a loading agent.
Table 1.
Attributes of replicated adhesives using Acacia gum: Hardness (HR15X), pH, and success rate
| Adhesive ingredients | Hardness (HR15X) | pH |
Frequency |
|||
|---|---|---|---|---|---|---|
| Acacia gum | Unheated adhesive | Heated adhesive | Successful adhesive | Failed adhesive | ||
| Gum no. 1 | 28 (37.3) 57 | 3.0–3.5 | 3.0–3.5 | 3.5–4.0 | 1 | 1 |
| Gum no. 2 | – | 4.0 | 4.0 | 5.0–5.5 | 3 | 1 |
| Gum no. 3 | – | 4.4 | 4.4 | – | 1 | 1 |
| Gum no. 1 plus synthetic Fe2O3 | 71 (80.5–93.5) 96 | 3.0–3.5 | 5.5–6.0 | 6.0 | 0 | 5 |
| Gum no. 2 plus synthetic Fe2O3 | – | 4.0 | 6.0 | 6.0 | 0 | 4 |
| Gum no. 1 plus yellow ochre no. 10 | 57 (65.2) 72 | 3.0–3.5 | 4.5–5.0 | 5.0–5.5 | 1 | 0 |
| Gum no. 2 plus yellow ochre no. 10 | – | 4.0 | 5.0 | 5.5 | 2 | 1 |
| Gum no. 2 plus yellow ochre no. 3 | – | 4.0 | – | – | 0 | 3 |
| Gum no. 1 plus red ochre no. 12 | 49 (51) 53 | 3.0–3.5 | 4.0–4.5 | 5.0–5.5 | 1 | 0 |
| Gum no. 2 plus red ochre no. 12 | – | 4.0 | 6.0 | 6.0 | 1 | 4 |
| Gum no. 2 plus red ochre no. 15 | – | 4.0 | 5.5–6.0 | 5.5–6.0 | 2 | 0 |
| Gum no. 3 plus red ochre no. 15 | – | 4.4 | – | – | 15 | 1 |
| Gum no. 1 plus red ochre no. 12 plus wax | – | 3.0–3.5 | 4.0–4.5 | 5.0–5.5 | 1 | 0 |
| Gum no. 2 plus red ochre no. 13 plus wax | 18 (32.3) 43 | 4.0 | 4.5 | 5.0 | 1 | 0 |
| Gum no. 3 plus red ochre no. 15 plus wax | – | 4.4 | – | – | 3 | 0 |
Adhesives were heated over fire before mounting tools and were later dehydrated near fire. See Table 2 for ochre attributes.
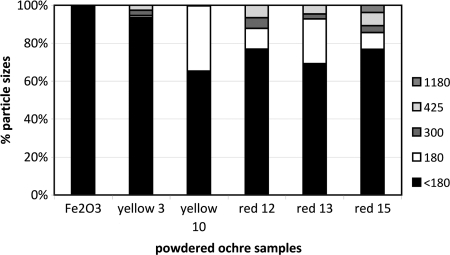
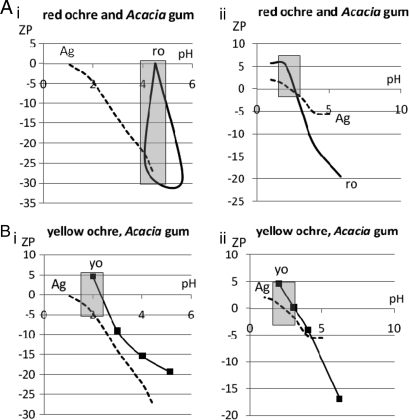
We report selected properties of replicated adhesives and/or their components: hardness, iron (Fe) and silica (Si) content, particle size, pH, and zeta potential (ZP)* (Tables 1 and 2 and Table S3; and Figs. 2 and 3). Ochre no. 15, a constituent of many successful adhesives, had both high Fe and Si contents (Table 2 and Table S2) and >20% particles in the size range 180-1180 μm (Fig. 2), and starting at pH 4.5 (when mixed with deionized water), its ZP was almost at zero charge (Table S3 and Fig. 3), implying potential for particle aggregation. Fire was an essential tool for dehydrating our adhesives; they dried within 4 hours near a controlled fire but took 6 days to dry in ambient temperatures (13).
Table 2.
Selected attributes of powdered ochre used in our experiments
| Sample | Munsell color | Geological type | Geographical origin | % Fe2O3 | % SiO2 |
|---|---|---|---|---|---|
| Synthetic Fe2O3 | 7.5R 4/6 red | – | – | 83.9 | 11.8 |
| No. 3 | 7.5YR 6/6 reddish yellow | Snuffbox shale | Ndwedwe quarry near Sibudu Cave | 15.5 | – |
| No. 10 | 10YR 7/8 yellow | ″ | ″ | 21.3 | 13.4 |
| No. 12 | 7.5 R 3/4 dusky red | ″ | ″ | 56.9 | 10.3 |
| No. 13 | 10R 4/4 weak red | ″ | ″ | 51.4 | – |
| No. 15 | 7.5R 4/6 red | Hematite | Waterberg | 57.6 | 33.7 |
Fe and Si readings were obtained by ICP-OES, except for no. 15, which was obtained by X-ray fluorescence.
Fig. 2.
Particle size analysis of ochre samples used in our experiments. Fe2O3 is synthetic hematite.
Fig. 3.
Comparison of ZP readings on red ochre no. 15, A. karroo gum, and yellow ochre no. 10. Ag, Acacia gum; ro, red ochre; yo, yellow ochre. Samples in Ai and Bi were mixed with 50 mL of d-water; samples in Aii and Bii were mixed with 50 mL of 0.05 M KCl. The gray shaded blocks show the pH and ZP at which maximum adhesion might be expected to occur.
Hardness seems unimportant to the success or failure of adhesives, because synthetic Fe2O3 adhesives were harder than any other adhesives, yet they were always unsuccessful (Table 1), whereas adhesives including beeswax were softer, yet they displayed desirable workability,† cohesiveness,‡ consistence,§ and plasticity.¶ Only a small amount of beeswax (for example, 15% by weight) was needed to achieve ideal plasticity and cohesiveness; larger amounts led to creep‖ and shrinkage.
Red ochres used for our adhesives contained more Fe than the yellow ochres (Table 2), so red seems a more reliable predictor of iron content. The associated energy-dispersive spectrometer (EDS) spectra (Fig. S1) of the red and yellow adhesives are typical of hematite and goethite profiles, respectively (28). All yellow ochre no. 3 adhesives with 15.5% Fe were unsuccessful, but synthetic hematite adhesives had 83.9% Fe and also failed, implying that high iron content alone cannot guarantee success. SiO2 content may be influential. A third of red ochre no. 15 comprised SiO2 (quartz, feldspars, mica, and kaolin) (Table 2), an inclusion partly derived from grains abraded from sandstone slabs during grinding of the ochre nodules (Fig. S2).
Mixed particle sizes seem critical to the success of compound adhesives (Fig. 2), and the production of modern industrial concrete can be used as an analogy to explain the importance of aggregates. Concrete is made by mixing Portland cement (comprising clinkered calcium carbonate, iron oxide, silica, and alumina that are ground together with gypsum to a powder with a high surface area) with water and fine and coarse aggregates, such as sand and stone chips (27). The cement-and-water mixture covers the surfaces of the sand and stone, and through hydration the mixture hardens to form concrete. Fine silt and clay particles must not occur in large quantities because these form a film around the sand and stone chips (27). Thus, the production of modern concrete depends on the correct proportions of sand and stone aggregate, and the same principle seems to apply to natural adhesive manufacture, where coarse grains must be included to provide, in microcosm, the equivalent of aggregates in concrete. Particle size analysis showed that all of the powdered ochres had high proportions of particles smaller than 180 μm (Fig. 2). Red ochre no. 15 was the only sample with grains of 1,180 μm (the equivalent of stone chips in concrete). All synthetic hematite particles were smaller than 180 μm, and these adhesives failed.
Chemical changes took place when iron products were added to Acacia gum. Gum samples that we used from different trees had pH readings between 3 and 4.4 (the former pH being 10 times more acidic than the latter). When ochre was added, the mixture became less acidic (Table 1). Preheating for 1.5 min before hafting raised the pH on some adhesives, whereas others remained unchanged. A pH change from 4.0 to 6.0 represents 2 orders of magnitude, whereas that from 3.0 to 6.0 is more dramatic, representing 3 orders of magnitude. However, the success or failure of our adhesives could not be predicted by the degree of pH change, even though pH affects precipitation, dissolution, and reprecipitation of iron (14), and even slight pH manipulations drastically alter the oxide or oxyhydroxide content and the ZP of an iron product (26).
In the ZP analysis, pH and ZP readings using water as a diluent were very different from those using KCl (Table S3 and Fig. 3). This difference underscores the sensitivity of natural products to small changes in pH or ionic strength. However, in all cases, the charge became more negative as solutions became basic, whereas charge became more positive with increasing acidity. Particularly noteworthy was the initial ZP, before acid addition, for red ochre no. 15 mixed with deionized water (Table S3 and Fig. 3). This ZP was close to zero charge, and it was strikingly different from ZP readings for the other products. The low value suggests contamination, because the usual point of zero charge (zpc) for hematite is 8.5–8.8 (28). Zpc/isoelectric point (iep) values are sensitive to impurities (28), and the most likely contaminant in ochre no. 15 is Si. Si lowers pH and zpc values, because quartz pH and zpc values are lower than those of iron oxides (28). When the pH of ochre no. 15 reached 4.5 after acid addition, the red ochre particle aggregation (and adhesion potential) would have been at its greatest. An apparent anomaly was the rise of pH in ochre no. 15 (diluted initially with d-water) with the constant addition of acid. This result might be explained by the increased solubility of Fe2O3 with the addition of acid. ZP could plausibly have played a role in the success of some adhesives, but this suggestion must remain a hypothesis for future testing, because ZP readings are not ordinarily taken on natural products, and the field remains unexplored.
Discussion and Conclusions
Compound adhesives combining plant gum and ochre have properties different from those of simple adhesives using only plant gum, and they would have had different uses. Factors involved during compound adhesive manufacture using ochre seem to have included the chemical change from acidic to less acidic pH, dehydration of the adhesive by using well-controlled temperature, electrostatic forces (measured by ZP) that may have contributed to adhesion, and the mechanical property of workability achieved through the addition of a coarse particle component and/or an agent, such as beeswax or fat. We do not yet have all of the answers for the choice of red ochre for some (but not all) MSA compound adhesives. It would have ensured relatively high iron content, but to overcome the disadvantages of product inconsistencies, artisans may have deliberately added sand grains to provide suitable aggregate and may have routinely forced dissolution/reprecipitation and dehydroxylation, so that the combined processes mechanically and chemically altered the adhesive to the desired state. Future work can build on our pioneering study, increase sample size, and improve methodology. It could investigate the hygroscopic properties of different ochres. It could also explore a colloidal model because organic acids and starch molecules adsorb on iron surfaces (14, 29, 30), enabling their use for purifying gases and fluids, treating mineral ores (31, 32, 33), and even creating edible iron supplements (34).
Hunters' lives depend on reliable weapons. This dependency would have been a powerful incentive in the past to create trustworthy adhesives for composite weapons. Our experiments intimate that by at least 70 ka (and earlier evidence may eventually be found at sites other than Sibudu) people were competent chemists, alchemists, and pyrotechnologists. We propose that these artisans were exceedingly skilled; they understood the properties of their adhesive ingredients, and they were able to manipulate them knowingly.
Although we have devoted much time to discussing the mechanical and chemical effects of adding ochre to plant gum for the creation of compound adhesives, we have done so to highlight the behavioral implications of this technology. We shall never know for sure whether the process of creating compound adhesive from disparate ingredients was regarded as symbolic in the past. However, our familiarity with compound adhesive manufacture from natural ingredients helps us make interpretations about the type of cognition that the early artisans must have had. Some birds and wasps also create compound adhesives, but they do so instinctively with simply coded operational sequences, “cognigrams,” in which the distance between problem and solution is far smaller than that demonstrated by the human action of making a composite hunting weapon (35). One obvious difference in human manufacture of compound glue is the use of pyrotechnology. Temperature control depends on understanding wood types, their moisture contents, and their propensity to form long-lasting coals. Vigilance is essential because our adhesives burned, or boiled to form air bubbles, when they were too close to the fire. Overdehydration caused loss of cohesiveness, whereas boiling adhesive created weakness.
The glue maker needs to pay careful attention to the condition of ingredients before and during the procedure and must be able to switch attention between aspects of the methodology. To hold many courses of action in the mind involves multitasking, which is one trait of modern human minds (2), notwithstanding that even today, some people find multilevel operations difficult. On-the-spot compensations have to be made for the capricious character of natural ingredients. Viscosity of Acacia gums varies, demanding different quantities of loading agent. Powdered ochres are also inconsistent: even when they are visually similar because of red staining by minute quantities of hematite, which has pervasive pigmenting capacity (14), they can be dissimilar with respect to Fe and Si percentages, particle size, pH, and ZP. Thus, ongoing evaluation and control of texture, viscosity, plasticity, and temperature is required; no set recipe or routine can guarantee a satisfactory adhesive product.
Mental flexibility is not the only complex attribute implied by our experiments. Artisans living in the MSA must have been able to think in abstract terms about properties of plant gums and natural iron products, even though they lacked empirical means for gauging them. Qualities of gum, such as wet, sticky, and viscous, were mentally abstracted, and these meanings counterpoised against ochre properties, such as dry, loose, and dehydrating. Simultaneously, the artisan had to think about the correct position for placing stone inserts on the hafts. Successful mental rotation requires advanced working memory capacity (36) and, in turn, complex cognition. Capacity for multilevel operations, abstract thought, and mental rotation are all required for the process of compound adhesive manufacture. Although fully modern behavior is presently recognizable relatively late in the MSA (37), the circumstantial evidence provided here implies that people who made compound adhesives in the MSA shared at least some advanced behaviors with their modern successors.
Materials and Methods
Acacia gum collected from Acacia karroo and Acacia tortilis trees fluctuated between being highly viscous, and dry and crystalline. Its pH varied between 3.0 and 4.4 (Table 1). Natural yellow and red ochres were collected from a Snuffbox Shale quarry in the vicinity of Sibudu Cave. Ochre no. 15 came from the Waterberg, Limpopo. Ochre was powdered by rubbing nodules on coarse, flat sandstone slabs (Sibudu roof-spall; Fig. S2) because such slabs with ochre stains have been found at Sibudu.
Three adhesive recipes are reported here: (i) Acacia gum alone (5 mL); (ii) Acacia gum (5 mL) mixed with either natural ochre powder (2.5 mL) or synthetic hematite powder (2.5 mL); and (iii) Acacia gum (5 mL) mixed with natural ochre powder (2.5 mL) and heated beeswax (1.2 mL).
All of our replications were conducted with open wood fires whose temperatures varied depending on the type and amount of wood used but did not exceed 726 °C. Temperatures were recorded by using an MT 632 thermometer (manufactured by MajorTech Pty Ltd) with a 50-cm-long thermocouple.
The adhesive was partially dehydrated on a stick rotated near fire for 1.5 min at a temperature that varied between 250 °C and 300 °C. The adhesive was then used to mount the stone insert to its wooden haft (Fig. S3), and the hafted tool was placed near the fire (60–100 °C) for 4 hours to dry and harden the adhesive. Each tool was rotated at 10-min intervals (more frequently with wet adhesive) and was moved closer or farther from the fire depending on the heat.
Samples of adhesives and/or ochre were analyzed as follows:
Particle-size analysis was conducted by weighing each powdered ochre sample on a digital balance, then sieving it through a stack of geological sieves (1,180, 425, 300, and 180 μm). Each component was weighed after sieving.
Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used for elements in Table 2. Nitric acid [5 mL (55%)], hydrofluoric acid [4 mL (40%)], and hydrochloric acid [0.5 mL (33%)] were added to each sample of iron powder (0.25 g). The samples were heated in a Multiwave 3000 V1.27 microwave oven (manufactured by Anton Paar) before transfer to the ICP-OES (manufactured by Spectro Genesis).
X-ray fluorescence spectrometry was used to characterize chemically the elements in Table S2.
EDS analysis was used for additional elemental analysis of adhesives. A spot-mode technique was performed on different areas (for example, featureless versus granular areas) of 6 adhesives. The spectra show peaks of elements (Fig. S1), but these are not an indicator of precise quantities of the elements represented; they correspond to the strengths of energy pulses emitted from the samples.
Replicated adhesives were pressed into molds when wet and were dried near an open fire before measuring their hardness with a Superficial Rockwell T Hardness tester (HR15X) (manufactured by Time Testing Instruments) with a 15-kg full load, 3-kg preload, and 6-mm steel ball indenter. Several measurements were taken from each sample, and outliers were excluded.
pH was measured by using a pH probe, pH indicator papers, and a Malvern Zetasizer Nano system Z3600 (for the samples submitted for ZP readings).
ZP was measured by using a Malvern Zetasizer Nano system Z3600 (manufactured by Malvern Instruments Ltd). Temperature was maintained at 25 °C. One set of samples used 0.05 M KCl as a diluent, and another identical set used deionized water. A total of 0.1 g of each sample was added to 50 mL of either 0.05 M KCl or deionized water. To acidify the solutions, 0.01 M HCl was used. Four samples were selected for ZP readings: A. karroo gum, red ochre no. 15, yellow ochre no. 10, and adhesive made from A. karroo gum and red ochre no. 15.
Each tool was used for chopping Ozoroa paniculosa branches for 5 min at a constant rate of 2 strokes per second to test the resilience of the adhesives. Adhesive was considered successful if it survived the 5-min task without breaking.
Supplementary Material
Acknowledgments.
M. Lombard (University of the Witwatersrand) provided the residue micrographs, S. Turner (University of the Witwatersrand) the X-ray fluorescence readings, and H. Divey (University of Cape Town, Cape Town, South Africa) the ZP readings. J. Hughes, T. McCarthy, and R. G. Roberts commented on an early draft of the paper. Research by L.W. was supported by the National Research Foundation and the University of the Witwatersrand.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 9544.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900957106/DCSupplemental.
ZP is electrostatic potential that, as it moves toward 0 mV, increases the possibility of particle aggregation. ZP is routinely used to predict the relative stability of aqueous colloidal samples. If ZP is >30 mV or less than −30 mV, the particles tend to repel each other, which increases the subsequent dispersion stability. As ZP moves toward 0 mV—the isoelectric point (iep)—the possibility of particle aggregation increases, leading to a reduction in the stability (25, 26).
Workability is the relative ease with which the wet adhesive mix can be placed and compacted on the haft. It is the result of a combination of intrinsic properties, such as cohesiveness, consistence, and plasticity. The wet adhesive must not be so sticky that it cannot be worked or so soft that it sags, or the constituents segregate when it is placed (27).
Cohesiveness, or “stickiness,” is the degree to which the wet adhesive clings together in a continuous mass, or to surfaces to which it has been applied (27).
Consistence is the stiffness of the mixture; that is, the degree to which it resists deformation of flow (27).
Plasticity is the degree to which the lump of adhesive remains in a continuous mass while being molded or extruded. Plasticity is not synonymous with consistence (27).
Creep is the increase in strain—that is, deformation—under an applied stress (27).
References
- 1.Amati D, Shallice T. On the emergence of modern humans. Cognition. 2007;103:358–385. doi: 10.1016/j.cognition.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Barnard PJ, Duke DJ, Byrne RW, Davidson I. Differentiation in cognitive and emotional meanings: an evolutionary analysis. Cogn Emot. 2007;21:1155–1183. [Google Scholar]
- 3.Mazza PPA, et al. A new Palaeolithic discovery: Tar-hafted stone tools in a European Mid-Pleistocene bone-bearing bed. J Archaeol Sci. 2006;33:1310–1318. [Google Scholar]
- 4.Boëda LE, et al. Bitumen as a hafting material on Middle Palaeolithic artefacts. Nature. 1996;380:336–338. [Google Scholar]
- 5.Ambrose SH. Chronology of the Later Stone Age and food production in East Africa. J Archaeol Sci. 1998;25:377–392. [Google Scholar]
- 6.Lombard M. Evidence of hunting and hafting during the Middle Stone Age at Sibudu Cave, KwaZulu-Natal, South Africa: A multi-analytical approach. J Hum Evol. 2005;48:279–300. doi: 10.1016/j.jhevol.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Lombard M. First impressions on the functions and hafting technology of Still Bay pointed artefacts from Sibudu Cave. S Afr Hum. 2006;18:27–41. [Google Scholar]
- 8.Lombard M. The gripping nature of ochre: The association of ochre with Howiesons Poort adhesives and Later Stone Age mastics from South Africa. J Hum Evol. 2007;53:406–419. doi: 10.1016/j.jhevol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Lombard M. Finding resolution for the Howiesons Poort through the microscope: Micro-residue analysis of segments from Sibudu Cave, South Africa. J Archaeol Sci. 2008;35:26–41. [Google Scholar]
- 10.Williamson BS. Middle Stone Age tool function from residue analysis at Sibudu Cave. S Afr J Sci. 2004;100:174–178. [Google Scholar]
- 11.Wallis L, O'Connor S. In: A Closer Look: Recent Australian Studies of Stone Tools. Fullagar R, editor. Sydney: Sydney Univ; 1998. pp. 149–178. [Google Scholar]
- 12.Clark JD. Interpretations of prehistoric technology from ancient Egyptian and other sources. Part 2: Prehistoric arrow forms as shown by surviving examples of traditional arrows of the San Bushmen. Paléorient. 1975;3:127–150. [Google Scholar]
- 13.Wadley L. Putting ochre to the test: Replication studies of adhesives that may have been used for hafting tools in the Middle Stone Age. J Hum Evol. 2005;49:587–601. doi: 10.1016/j.jhevol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Schwertmann U, Cornell RM. Iron Oxides in the Laboratory: Preparation and Characterization. Cambridge, MA: Wiley-VCH; 1991. [Google Scholar]
- 15.Godfrey-Smith DI, Ilani S. Past thermal history of goethite and hematite fragments from Qafzeh Cave deduced from thermal activation characteristic of the 110 °C TL peak of enclosed quartz grains. Rev Archeom. 2004;28:185–190. [Google Scholar]
- 16.Sievers C, Wadley L. Going underground: Experimental carbonization of fruiting structures under hearths. J Archaeol Sci. 2008;35:2909–2917. [Google Scholar]
- 17.Asmussen B. Intentional or incidental thermal modification? Analysing site occupation via burned bone. J Archaeol Sci. 2008;36:528–536. [Google Scholar]
- 18.Wadley L. A taphonomic study of ochre demonstrates post-depositional color transformations. J. Taphonomy. 2009 in press. [Google Scholar]
- 19.Helwig K. A note on burnt yellow earth pigments: Documentary sources and scientific analysis. Stud Conserv. 1997;42:181–188. [Google Scholar]
- 20.van Wyk BE, van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa. Pretoria, South Africa: Briza; 1997. [Google Scholar]
- 21.Goldberg P, Macphail RI. Practical and Theoretical Geoarchaeology. Oxford: Blackwell; 2006. [Google Scholar]
- 22.Santos LD, Brandao PRG. Morphological varieties of goethite in iron ores from Minas Gerais, Brazil. Miner Eng. 2003;16:1285–1289. [Google Scholar]
- 23.Churms SC, Merrifield EH, Stephen AM. New aspects of the molecular structure of Acacia karroo gum shown by Smith degradations. S Afr J Chem. 1983;36:149–152. [Google Scholar]
- 24.Hughes JC, Solomon A. A preliminary study of ochres and pigmentaceous materials from KwaZulu-Natal, South Africa: Towards an understanding of San pigment and paint use. Natal Mus J Hum. 2000;12:15–31. [Google Scholar]
- 25.Zhang Z, Boxall C, Kelsall GH. Photoelectrophoresis of colloidal iron oxide: Hematite (α-Fe2O3) Colloids Surface A. 1993;13:145–163. [Google Scholar]
- 26.Addis BJ, Owens G. Fulton's Concrete Technology. Midrand, South Africa: Cement and Concrete Institute; 2001. [Google Scholar]
- 27.Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Weinheim, Germany: Wiley-VCH; 2003. [Google Scholar]
- 28.Bremmell KE, Scales PJ. Adhesive forces between adsorbed anionic polyelectrolyte layers in high ionic strength solutions. Colloids Surface A. 2004;247:19–25. [Google Scholar]
- 29.Kallay MN, Preočanin T, Marković J, Kovačević D. Adsorption of organic acids on metal oxides: Application of the surface potential measurements. Colloids Surface A. 2007;306:40–48. [Google Scholar]
- 30.Ji YQ, Black L, Koster R, Janek M. Hydrophobic coagulation and aggregation of hematite particles with sodium dodecylsulfate. Colloids Surface A. 2007;298:235–244. [Google Scholar]
- 31.Ferreiro EA, de Bussetti SG. Thermodynamic parameters of adsorption of 1,10-phenanthroline and 2,2′-bipyridyl on hematite, kaolinite and montmorillonites. Colloids Surface A. 2007;301:117–128. [Google Scholar]
- 32.Li LY, Rutherford GK. Effect of bauxite properties on the settling of red mud. Int J Miner Process. 1996;48:169–182. [Google Scholar]
- 33.Somsook E, et al. Interactions between iron(III) and sucrose, dextran, or starch in complexes. Carbohydr Polym. 2005;61:281–287. [Google Scholar]
- 34.Haidle MN. Tübingen, Germany: Univ Tübingen; 2006. Menschen–Denken–Objekte. Zur problem-lösung-distanz als kognitionsaspekt im werkzeugverhalten von Tieren und im laufe der menschlichen evolution. PhD dissertation. [Google Scholar]
- 35.Kane MJ, et al. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- 36.Klein RG. Out of Africa and the evolution of human behavior. Evol Anthropol. 2008;17:267–281. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.