Abstract
Lovelock and Whitfield suggested in 1982 that, as the luminosity of the Sun increases over its life cycle, biologically enhanced silicate weathering is able to reduce the concentration of atmospheric carbon dioxide (CO2) so that the Earth's surface temperature is maintained within an inhabitable range. As this process continues, however, between 100 and 900 million years (Ma) from now the CO2 concentration will reach levels too low for C3 and C4 photosynthesis, signaling the end of the solar-powered biosphere. Here, we show that atmospheric pressure is another factor that adjusts the global temperature by broadening infrared absorption lines of greenhouse gases. A simple model including the reduction of atmospheric pressure suggests that the life span of the biosphere can be extended at least 2.3 Ga into the future, more than doubling previous estimates. This has important implications for seeking extraterrestrial life in the Universe. Space observations in the infrared region could test the hypothesis that atmospheric pressure regulates the surface temperature on extrasolar planets.
Keywords: evolution, Gaia, greenhouse, habitability
The Earth is inhabitable largely because its atmosphere contains 2 principal greenhouse gases (water vapor and CO2) that serve as a blanket to trap heat emitted from the surface; these regulate the global mean temperature within the range in which life can evolve. If such an atmosphere were absent, the global mean temperature would be only −18 °C (1), and life, which relies on liquid water, would not exist. The standard model of stellar evolution (2) suggests that the solar luminosity was only 70% of the current value when the Sun formed, and has been steadily increasing to its current value over the past 4.6 billion years. This implies that the global mean temperature should have been increasing with time. However, the geological record indicates that our planet has stayed well within the inhabitable range of ≈0–50 °C since the Archean (3), aside from a few possible “Snowball Earth” events (4, 5). Climate models also suggest that the partial pressure of CO2 at the surface (PCO2) 3,500 Ma ago was as large as 7,000 Pa and has declined to the preindustrial value of 28 Pa (6). Had PCO2 remained unchanged at the level of 7,000 Pa, the present-day surface temperature would have been much more than 50 °C, the upper limit for most eukaryotic life. Walker et al. (7) suggested abiotic carbonate–silicate weathering as a mechanism that reduces the atmospheric CO2, but it was Lovelock and Whitfield (3) who first proposed biologically enhanced silicate weathering as the primary mechanism regulating the temperature throughout the history of the Earth. The model proposed by Lovelock and Whitfield (3) suggested that PCO2 would drop to ≈15 Pa, the lower limit for photosynthesis by C3 plants, in ≈100 Ma, implying the end of the photosynthetic biosphere. This idea was further developed by Caldeira and Kasting (8) with detailed calculations including C4 plants that can use CO2 at pressures as low as 1 Pa. As the solar luminosity increases, the partial pressure of CO2 should drop to 15 Pa in 0.5 billion years or gigaannum (Ga) and to 1 Pa in 0.9 Ga, eventually reaching a runaway greenhouse (9) that renders the Earth's surface uninhabitable. In their model, a saturated atmosphere was assumed, which allows the positive feedback by water vapor as the Earth's surface warms up. More elaborate models that include biological and geodynamic processes have also been developed (10, 11), but estimates for the life span of the biosphere remain at ≈1 Ga.
All of these previous studies focused on the greenhouse effect due to direct absorption by atmospheric species such as water vapor and CO2, whose radiative forcings are ≈80 Wm−2 and ≈30 Wm−2, respectively. However, atmospheric pressure also plays a critical role in the greenhouse effect through broadening of the infrared absorption lines of these gases by collisional interaction with other molecules (mainly N2 and O2 in the present atmosphere) (1). In other words, if the total atmospheric pressure were lower, the climate forcing of greenhouse gases would be smaller, the magnitude of the greenhouse effect would be less, and the global mean temperature would drop (see Fig. 2 and later discussions). If, via natural or artificial processes, it were possible to reduce the Earth's atmospheric pressure, the life span of the biosphere would be extended.
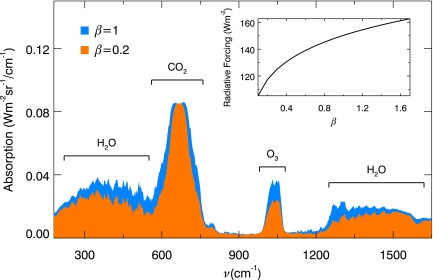
Fig. 2.
Absorption spectra at the top of the atmosphere in the present-day Earth for β = 1 (blue) and 0.2 atm (orange), showing the effect of pressure broadening. The spectral resolution is 20 cm−1. The major species responsible for absorption are labeled. (Inset) The total radiative forcing due to the absorption by greenhouse gases as a function of β.
Geochemical and geobiological processes may have varied atmospheric pressure throughout the history of the Earth (12). Biology regulates overall atmospheric pressure in 2 related but distinct ways. First, the≈2 × 104 Pa of oxygen in the present atmosphere is maintained directly by oxygenic photosynthesis: photosystem-II, operating in green plants, algae, and cyanobacteria. With the possible exception of O2 produced by the sudden release of peroxides accumulated in the ice caps of a Snowball Earth (5), no other mechanism—biotic or abiotic—is known to be capable of maintaining even trace amounts of this gas in the Earth's atmosphere (see ref. 4 for a recent discussion).
The second pressure component in the Earth's atmosphere that interacts with biology is the reservoir of gaseous N2; at nearly 78% volume mixing ratio (vmr), it has the largest influence on pressure broadening of the greenhouse gas absorption bands. The atmospheric nitrogen budget is ultimately tied to the oceanic primary productivity and the subsequent burial of organic material in the sediments. In the present ocean, organic matter is incorporated into living organisms in the stoichiometric Redfield ratio of 106:16:1 for C, N, and P (13). Thus, the C/N ratio is ≈6.6. But when the organic carbon is buried in the marine sediments, its C/N ratio is much larger because N, an essential nutrient, has been stripped for recycling (see, e.g., chapter 11 of ref. 14). There are measurements of nitrogen in sediments, hard crustal rocks, and the mantle (15–17): The estimated amounts in the atmosphere, crust, and mantle are 2.8, 0.4 ± 0.2, and 5.5 ± 1.9, respectively, in units of 1020 mol of N (15). These measurements show that it is possible to sequester on the order of an atmosphere of N2 in the crust and mantle. The higher N/rock ratios seen in the data are believed to be associated with biology.
Thus, natural processes may have caused variations in atmospheric N2 in the history of the Earth, a result that is testable by measurements. For instance, basalt vesicularity has been used to infer atmospheric paleopressure and hence paleoelevation by using a standard atmosphere that is the same as the present one (18). Recently, measurement of atmospheric N2 in the Archean period has also been proposed (29).
Model and Results
Given the potential for biological control of both oxygen and nitrogen, we cannot assume that the abundance of either of these gases has remained constant over geologic time. Here, we present a model to estimate how much the life span of the biosphere might be extended by regulating atmospheric pressure. We emphasize that the model to be presented is maximally simplified and that it aims only to estimate the first-order effect due to the pressure broadening. We shall discuss the shortcomings of the model in the final paragraph of this section. We assume that during the next 900 million years, the atmospheric pressure is kept constant at the present value (105 Pa). Following Caldeira and Kasting (8), as the solar luminosity increases, the surface temperature rises to 300 K, and PCO2 drops logarithmically to 1 Pa. At this time, the column abundances of CO2 and other trace gases including water vapor remain constant such that C4 plants can subsist. In our model, the atmospheric pressure is lowered to maintain the surface temperature Tsurf at 300 K. For a given solar luminosity, the effective temperature Teff of the Earth at radiative equilibrium can be obtained from,
where a ≈ 0.3 is the planetary albedo and S is the solar luminosity measured at 1 AU, which is a function of time given by ref. 8
where S0 = 1,368 Wm−2 is the current value of the solar luminosity (19). This incoming solar energy must be balanced by the outgoing longwave radiation, defined through the Stefan–Boltzmann law (1, 20):
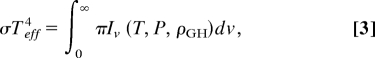 |
where σ = 5.67 × 10−8 WmK−4 is the Stefan-Boltzmann constant. Iv is the outgoing radiation measured at the top of the atmosphere at wave number v, which is a function of the vertical profiles of temperature T(z), pressure P(z), and the mixing ratios ρGH(z) of the greenhouse gases; it is related to atmospheric pressure due to the pressure broadening effect described above. Thus, given the solar luminosity S(t) at a particular time t and the profiles ρGH(z) and T(z) with Tsurf = T(z = 0), one aims to solve for the function P(z) such that Eqs. 1 and 3 are equal. For simplicity, we assume that P(z) = βPref(z), i.e., the pressure profile is scaled as a whole, for some reference pressure profiles such that the problem is reduced to solving for the scalar constant β.
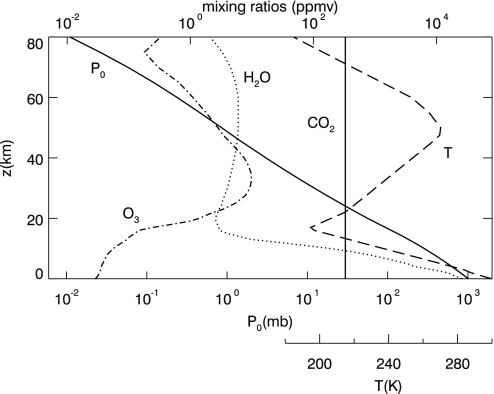
Iv is calculated from the MODTRAN radiative transfer code (21) by using the atmospheric profiles from the U.S. Standard Tropical Atmosphere data (22), including Pref(z) (the reference profile), ρGH(z) and T(z), where the surface temperature Tsurf ≈ 300 K (Fig. 1). We assume that T(z) remains constant after 0.9 Ga. Fig. 2 illustrates the effect of pressure broadening on the absorption features due to the major greenhouse gases. The absorption spectra have been averaged to a resolution of 20 cm−1. It is evident that the amount of absorption is reduced at lower pressure. The area under the absorption spectra can be integrated to give the total radiative forcing, which is shown in the Fig. 2 Inset as a function of β. As the solar luminosity evolves, we solve for the scaling factor β such that the equality between Eqs. 1 and 3 holds and that the energy balance is achieved:
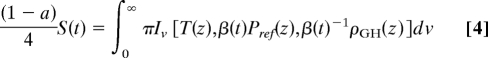 |
Here, β(t), which is time dependent as is S(t), is the only parameter to be determined. Except for ozone, which is photochemically produced from the ambient oxygen gas whose concentration scales with the atmospheric pressure, ρGH(z) is inversely scaled by β(t)−1 such that the total columns of major greenhouse gases such as H2O and CO2 are kept constant.
Fig. 1.
U.S. Standard Tropical Atmosphere profiles used in this work. Note that 1 mb = 100 Pa.
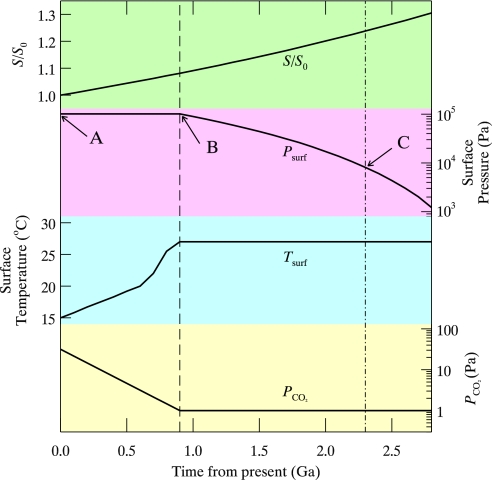
The derived evolution of the surface pressure, given by β(t)Pref(z = 0), is shown in Fig. 3. It drops to 3,500 Pa at ≈2.5 Ga, when the atmosphere consists mostly of water vapor. When the atmosphere becomes aquatic, most plants and other aerobic forms of life will be wiped out, marking the end of the aerobic biosphere. Only bacteria that are capable of carrying out anaerobic metabolisms can survive and continue the life processes. Thus, 2.5 Ga serves as a biological lower limit for the life span of the biosphere. This is a coarse estimate in that we have neglected the collision-induced absorption of water vapor. It is natural to ask whether the Earth's surface pressure will in fact decrease according to the previous condition. As pointed out by Walker (p 118 in ref. 23), nitrogen fixation by biology acts to naturally draw down the nitrogen content of the atmosphere through the process of organic matter burial. We can estimate an upper limit on the efficacy of this process as follows. The carbon isotope record (24) reveals that organic matter has accounted for ≈20% of the carbon sinks at the Earth's surface in the last 3.5 Ga. Considering that a vast reservoir (≈60 bars CO2 equivalent) of carbonates exists in sediments, this implies a reservoir of organic matter (≈10 bars CO2 equivalent) including nitrogen, which, with a Redfield ratio of C/N ≈ 6, would imply 1 bar of N2 having been removed from the atmosphere over the past 4 Ga, a rate that is comparable with that needed to offset the effects of increasing solar luminosity.
Fig. 3.
The evolution of surface pressure and temperature and the partial pressure of CO2 in the model as the solar luminosity evolves in the next 2.8 Ga from present. Before 0.9 Ga (dashed line), the quantities evolve as described in Caldeira and Kasting (8). After 0.9 Ga, the surface temperature and the partial pressure of CO2 at the surface (PCO2) are kept constant while the atmospheric pressure is lowered to compensate the temperature increase due to the increase in solar luminosity (see Models and Results). At ≈2.3 Ga (dash-dotted line), runaway greenhouse occurs, signaling the ultimate fate of the biosphere.
However, it is difficult to extend the life span of the biosphere much beyond 2 Ga physically because the runaway greenhouse limits the stability of the atmosphere against loss of water vapor from the planet. In the present atmosphere, loss of water is mitigated by the cold tropopause. Therefore, even though the mixing ratio of H2O is ≈1% near the surface, this value drops to ≈10−6 near the tropopause, effectively trapping the H2O in the troposphere. However, this cold trap would fail if the atmosphere were composed of ≈40% H2O. In this case the moist lapse rate would be insufficient to cause cold trapping at the tropopause; H2O would be abundant in the upper atmosphere, where it would be dissociated by UV radiation, followed by escape to space (9). This is probably the explanation of why Venus is so dry compared with Earth, having lost an ocean of H2O via failure of the cold trap. Referring to Fig. 3, Tsurf ≈ 27 °C implies PH2O ≈ 3,500 Pa. The runaway greenhouse threshold PRG may be estimated from PH2O/PRG ≈ 0.4, from which we obtain PRG ≈ 8,900 Pa or 0.08 atm for the atmosphere to be stable against escape. The pressure must not fall below PRG, which according to our model occurs 2.3 Ga from present. (dashed–dotted line in Fig. 3).
In the above calculations, we have neglected biological or geological feedback mechanisms like those in refs. 10 and 11). For example, as the Earth's mantle cools down in time, the volcanic source of atmospheric CO2 may accordingly drop (10). In the absence of feedbacks, this would imply a lower level of atmospheric CO2 in steady state than the constant value of 10 Pa assumed in Fig. 3 after 0.9 Ga. This difficulty arises because we have considered the fall of CO2 and the pressure loss separately in different time domains, whereas in reality, they will operate simultaneously, the modeling of which requires additional research.
Discussion
Such a prolonged life span for the aerobic biosphere has implications for the search for life on extrasolar planets and the question of intelligent life in the Universe (25). The presence of oxygen-derived ozone (O3) in the atmospheres of planets is considered to be a biomarker that enables remote identification of the presence of life. Because the Earth has already expressed this signature for 2.3 Ga, the continued persistence of the photosynthetic biosphere for 2.3 Ga into the future implies that the Earth will be identifiable as an inhabited planet for nearly half the total lifetime of the Sun, an important point to consider in the search for life on extrasolar planets.
In general, inert gases besides N2 may provide significant pressure broadening in the atmospheres of exoplanets. Origin scenarios allow for a wide range of atmospheric masses and compositions for forming planets. Thus, given the amount of greenhouse gases in the atmosphere, the type of star the planet is next to and the orbital distance of the planet from the star, one can anticipate that the parameter that determines whether a planet is habitable is atmospheric pressure. Indeed, this is the major reason why present-day Mars is not inhabitable.
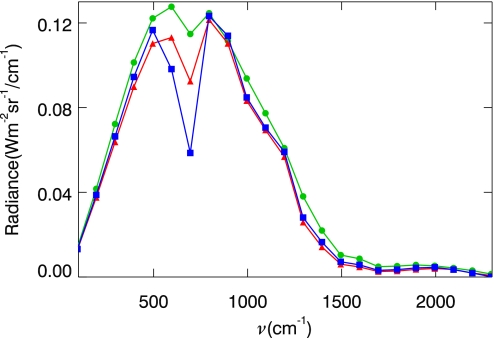
Remote sensing measurements using low-resolution infrared spectra have provided convincing evidence of the habitability of extrasolar planets (26) and the search continues. In the Earth's atmosphere, the dominant absorption band in the infrared region at ≈700 cm−1 is due to stratospheric CO2, whose bandwidth is ≈200 cm−1 (Δv/v ≈ 0.28). Thus, this CO2 band is the most conspicuous signature to examine the pressure broadening effect in the atmosphere of an Earth-like extrasolar planet. Fig. 4 shows the IR spectra of the Earth's atmosphere corresponding to the 3 stages shown in Fig. 3: (A) The present atmosphere (330 ppm CO2 at 1 atm); (B) The atmosphere 0.9 Ga after present as predicted by Caldeira and Kasting (8) (10 ppm CO2 at 1 atm); and, (C) The atmosphere ≈2.3 Ga after present as predicted by the present authors (10 ppm CO2 at 0.08 atm). In Stage A, the atmosphere is simulated with a water vapor abundance half that in the other 2 to account for a low vapor pressure due to the 10 K difference in the surface temperatures. With a resolution of Δv ≈ 100 cm−1(Δv/v ≈ 0.14), the absorption band at ≈700 cm−1 is clearly resolved. The absorption is reduced by half when CO2 drops from 330 to 10 ppm; it is reduced by another half when the pressure drops by a factor of 10. Differences in the water vapor absorption are also apparent. However, these absorption features cannot be distinguished from those in the current atmosphere (e.g., the Spitzer/IRS instrument has a resolution Δv/v ≈ 0.5 near 700 cm−1) unless the spectral resolution is at least twice as fine as the bandwidth of CO2. This poses a challenge for future space technology.
Fig. 4.
The emission spectra of the Earth's atmosphere corresponding to the 3 stages shown in Fig. 3. Blue: Stage A with 330 ppm CO2 under 1 atmospheric pressure; red: Stage B with 10 ppm CO2 under 1 atmospheric pressure; and, green: Stage C with 10 ppm CO2 under 0.08 atmospheric pressure.
Model Summary
The MODTRAN radiative transfer code has been used in this study (21). It has a moderate spectral resolution for wavelengths extending from thermal infrared to UV regions and it uses spherical geometry to compute the radiances. It solves the radiative transfer equations by the discrete ordinate method (27). We simulate the outgoing longwave radiation at the top of the atmosphere. The 1 cm−1 statistical band model (1) is used, where the molecular band parameters are derived from the HITRAN database. The Voigt equivalent width is directly evaluated from the exact expression for finite spectral bands (28).
Acknowledgments.
We thank D. Feldman, V. Natraj, R.-L. Shia, and C. Parkinson for reading the manuscript. We also thank Prof. Norm Sleep and 2 anonymous reviewers for their insightful suggestions. K.F.L. and Y.L.Y. were supported by National Aeronautics and Space Administration Grant NNG06GF33G and the Virtual Planetary Laboratory at the California Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Goody RM, Yung YL. Atmospheric Radiation: Theoretical Basis. 2nd Ed. Oxford: Oxford Univ Press; 1989. [Google Scholar]
- 2.Gough DO. Solar interior structure and luminosity variations. Sol Phys. 1981;74(1):21–34. [Google Scholar]
- 3.Lovelock JE, Whitfield M. Life-span of the biosphere. Nature. 1982;296(5857):561–563. [Google Scholar]
- 4.Kopp RE, Kirschvink JL, Hilburn IA, Nash CZ. Was the Paleoproterozoic Snowball Earth a biologically-triggered climate disaster? Proc Natl Acad Sci USA. 2005;102:11131–11136. doi: 10.1073/pnas.0504878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang MC, Hartman H, Kopp RE, Kirschvink JL, Yung YL. Production of hydrogen peroxide in the atmosphere of a Snowball Earth and the origin of oxygenic photosynthesis. Proc Natl Acad Sci USA. 2006;103(50):18896–18899. doi: 10.1073/pnas.0608839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen T, Cess RD, Ramanathan V. Enhanced CO2 greenhouse to compensate for reduced solar luminosity on early Earth. Nature. 1979;277(5698):640–642. [Google Scholar]
- 7.Walker JCG, Hays PB, Kasting JF. A negative feedback mechanism for the long-term stabilization of Earth's surface temperature. J Geophys Res Oceans. 1981;86(C10):9776–9782. [Google Scholar]
- 8.Caldeira K, Kasting JF. The life-span of the biosphere revisited. Nature. 1992;360(6406):721–723. doi: 10.1038/360721a0. [DOI] [PubMed] [Google Scholar]
- 9.Ingersoll A. Runaway greenhouse—A history of water on Venus. J Atmos Sci. 1969;26(6):1191–1198. [Google Scholar]
- 10.Franck S, et al. Reduction of biosphere life span as a consequence of geodynamics. Tellus B. 2000;52(1):94–107. [Google Scholar]
- 11.Lenton TM, von Bloh W. Biotic feedback extends the life span of the biosphere. Geophys Res Lett. 2001;28(9):1715–1718. [Google Scholar]
- 12.Hart MH. Evolution of atmosphere of Earth. Icarus. 1978;33(1):23–39. [Google Scholar]
- 13.Emerson SR, Hedges JI. Chemical Oceanography and the Marine Carbon Cycle. Cambridge, UK: Cambridge Univ Press; 2008. [Google Scholar]
- 14.Burdige DJ. Geochemistry of Marine Sediments. Princeton: Princeton Univ Press; 2006. [Google Scholar]
- 15.Marty B, Dauphas N. The nitrogen record of crust-mantle interaction and mantle convection from Archean to present. Earth Planet Sci Lett. 2003;206(3–4):397–410. [Google Scholar]
- 16.Papineau D, Mojzsis SJ, Karhu JA, Marty B. Nitrogen isotopic composition of ammoniated phyllosilicates: Case studies from Precambrian metamorphosed sedimentary rocks. Chem Geol. 2005;216(1–2):37–58. [Google Scholar]
- 17.Watenphul A, Wunder B, Heinrich W. High-pressure ammonium-bearing silicates: Implications for nitrogen and hydrogen storage in the Earth's mantle. Am Miner. 2009;94(2–3):283–292. [Google Scholar]
- 18.Sahagian D, Proussevitch A. Paleoelevation measurement on the basis of vesicular basalts. Mineral Geochem. 2007;66:195–213. [Google Scholar]
- 19.Woods T, et al. Solar Irradiance Variability during the SORCE Mission. The Earth Observer. 2006;18(4):8–14. [Google Scholar]
- 20.Ackerman SA, Frey RA, Smith WL. Radiation budget studies using collocated observations from advanced very high-resolution radiometer, high-resolution infrared sounder/2, and earth radiation budget experiment instruments. J Geophys Res Atmos. 1992;97(D11):11513–11525. [Google Scholar]
- 21.Kneizy FX, et al. The MODTRAN 2/3 Report and LOWTRAN 7 Model. Waltham, MA: Phillips Lab, Geophysics Directorate, PL/GPOS, Hanscombe AFB; 1996. [Google Scholar]
- 22.Anderson GP, Clough SA, Kneizys FX, Chetwynd JH, Shettle EP. Tech Rep AFGL-TR-86-0110, AFGL (OPI) Waltham, MA: Phililips Lab, Hanscom AFB; 1986. AFGL atmospheric constituent profiles (0–120 km) [Google Scholar]
- 23.Walker JCG. Evolution of the Atmosphere. New York: Macmillan; 1977. p. 318. [Google Scholar]
- 24.Schidlowski M. Application of stable carbon isotopes to early biochemical evolution on Earth. Annu Rev Earth Planet Sci. 1987;15:47–72. [Google Scholar]
- 25.Beichman CA, Woolf NJ, Lindensmith CA, editors. The Terrestrial Planet Finder. Pasadena, CA: JPL Publication; 1999. [Google Scholar]
- 26.Tinetti G, et al. Water vapour in the atmosphere of a transiting extrasolar planet. Nature. 2007;448(7150):169–171. doi: 10.1038/nature06002. [DOI] [PubMed] [Google Scholar]
- 27.Stamnes K, Tsay SC, Wiscombe W, Jayaweera K. Numerically stable algorithm for discrete-ordinate-method radiative-transfer in multiple-scattering and emitting layered media. Appl Opt. 1988;27(12):2502–2509. doi: 10.1364/AO.27.002502. [DOI] [PubMed] [Google Scholar]
- 28.Berk A, Bernstein LS, Robertson DC. MODTRAN: A Moderate Resolution Model for LOWTRAN 7, Report GL-TR-89-0122. Waltham, MA: Phillips Lab, Geophysics Directorate, Hanscom AFB; 1989. [Google Scholar]
- 29.Schirber M. Measuring the weight of ancient air. [Accessed October 06, 2008];Astrobiology Magazine. 2008 Available at http://www.astrobio.net/news/