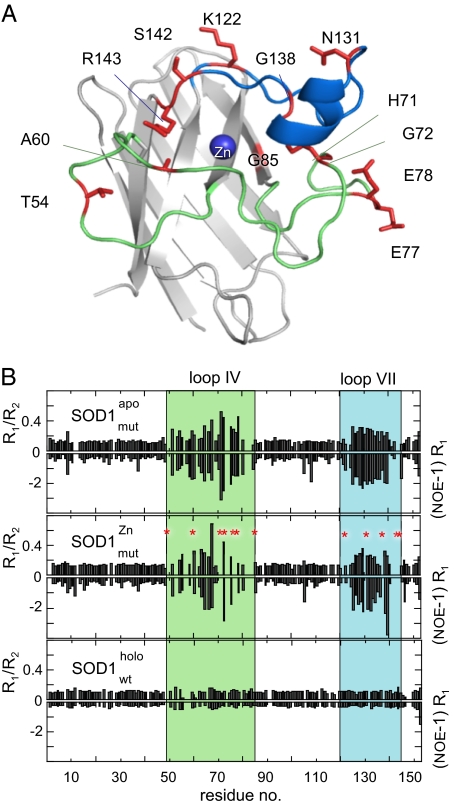
Fig. 5.
NMR shows that loop IV and loop VII of the misligated SOD1 monomer exhibit a high degree of backbone dynamics at both fast and intermediate time scales. (A). Structural representation of the SOD1 monomer showing the residues (red) in loops IV and VII that display increased dynamic complexity upon misligation of Zn. The rest of loops IV and VII retain apo-like dynamics. (B) Increased dynamics in the intermediate time scale is indicated by increased values of R1/R2 and increased fast dynamics as increased negative values of (NOE-1)R1. Apo SOD1 with mutated Zn ligands (SOD1mutapo) shows a dynamic pattern that is very similar to that of the apo state of the wild-type monomer. Upon misligation of Zn to the Cu site (SOD1mutZn) the resonances from some residues in loops IV and VII becomes line-broadened beyond detection (red). In the fully metallated wild-type monomer (SOD1pwtholo) (35) loops IV and VII are as fixed as the rest of the protein.