Abstract
Purpose:
To review the English language publications addressing the effect of intravitreal bevacizumab (IVB) injection in a variety of eye conditions and analyze the data where possible.
Methods:
Examination of data obtained using a Pubmed literature search conducted mid May 2007 with the keywords “intravitreal bevacizumab”.
Results:
A dose of 1.25 mg was used in 89.5% of 965 age-related macular degeneration (ARMD) cases with 47% receiving intravitreal bevacizumab as primary therapy. In 829 patients receiving repeated doses of 1.25 mg the mean logMAR best-corrected visual acuity (BCVA) improved from 0.88 at baseline to 0.74 at 4–6 weeks, 0.71 at 8–10 weeks, 0.67 at 12–14 weeks and 0.86 at >14 weeks. Mean central retinal thickness (CRT) decreased by 83.71 μm at 4–6 weeks, 79.52 μm at 8–10 weeks, 92.46 μm at 12–14 weeks, and 75.64 μm at >14 weeks respectively. In 64 patients receiving IVB for retinal vein occlusion (RVO) mean logMAR BCVA decreased from 1.21 at baseline to 0.83 and 0.82 at 4 and 12 weeks respectively. Mean CRT decreased from 635.97 μm at baseline to 320.06 μm and 346.27 μm at 4 and 12 weeks. Favorable responses have been reported in various other conditions.
Conclusions:
Current evidence suggests that intravitreal bevacizumab, alone or as an adjunct to conventional therapy, has a beneficial effect in various neovascular and edematous retinal conditions and is well tolerated in the short term.
Keywords: intravitreal, bevacizumab, review
Introduction
Bevacizumab (Avastin®; Genentech, South San Francisco, CA, USA) is a monoclonal antibody that binds all isomers of vascular endothelial growth factor (VEGF). It was approved by the US Food and Drug Administration (FDA) in February 2004 for intravenous treatment of metastatic colorectal cancer.
Intravenous administration of bevacizumab produced significant improvement in visual acuity and reduction of central retinal thickening in patients with age-related macular degeneration (ARMD) (Michels et al 2005; Moshfeghi et al 2006).
Choroidal neovascularization (CNV) due to pathological myopia was also suppressed by systemic bevacizumab (Nguyen et al 2005). The problem with systemic therapy, however, lay in the potential side-effect profile, with thromboembolic events such as transient ischemic attacks, strokes, angina, and myocardial infarction having been reported in oncology patients (Shah et al 2005). Theoretically, decreasing the dose of bevacizumab would decrease the potential risk of adverse effects, and one method to achieve this reduction is to inject the drug directly into the vitreous cavity. Multiple intravitreal anti-VEGF antibody injections not only prevented iris neovascularization in primate eyes but also did not induce inflammation (Adamis et al 1996), thus the decision was made to offer off-label intravitreal bevacizumab (IVB) to a patient with neovascular ARMD who continued to lose vision despite photodynamic therapy (PDT) with intravitreal triamcinolone and pegaptanib injections (Rosenfeld et al 2005). Within 1 week the subretinal fluid in the injected eye had resolved and no inflammation was observed.
Since then, off-label intravitreal bevacizumab use has been reported in a variety of neovascular conditions and this article reviews and analyzes the recent published results.
Methods
A literature search was conducted on Pubmed in May 2007 using the keywords “intravitreal” and “bevacizumab”. English-language publications were selected that reported the effect of intravitreal bevacizumab in a variety of neovascular ocular conditions. The results pertaining to separate entities were grouped together and analyzed, where possible, after converting all reported visual acuities to their logMAR equivalent. In the case of ETDRS letters being reported this was done by using the formula: logMAR = 1.7 – (0.02) (ETDRS letter score) (Cotter et al 2003). Weighted means were calculated for the subgroups in order to better quantify the overall results of the different studies but statistical significance could not be determined since critical data were not always available in the studies reviewed.
Results
Neovascular ARMD
The beneficial effect reported by Rosenfeld et al (2005) prompted several investigators to study off-label IVB in neovascular ARMD patients.
A dose of 1.0 mg bevacizumab was used for the first intravitreal injection but, in subsequent reports, doses used ranged between 1.0 mg and 2.5 mg. A dose of 1.25 mg was used in 864 (89.5%) of the 965 reported ARMD cases. Costa et al (2006) demonstrated a dose-related change in best-corrected visual acuity (BCVA) at week 12 in their study where vision improved by +0.3 ETDRS line after a dose of 1.0 mg, by +0.6 line after 1.5 mg, and by +1.0 line after 2.0 mg of IVB (p = 0.02). In 53% of 926 patients IVB was used after conventional treatment options did not bring about the desired result and 47% therefore received IVB as primary therapy.
Single 2.5 mg of bevacizumab injection
Table 1 shows the results of 2 studies using 2.5 mg of bevacizumab for intravitreal injection. In both instances the best corrected visual acuity (BCVA) and central retinal thickness (CRT) measured with ocular coherence tomography (OCT) showed a marked improvement at 4 weeks post-injection (Table 1).
Table 1.
Effect of a single intravitreal injection of 2.5 mg bevacizumab on median BCVA and CRT at 0 and 4 weeks in patients with neovascular ARMD
| Authors | n = | Median logMAR BCVA at 0 wks | Median logMAR BCVA at 4 wks | Median CRT in μm at 0 wks | Median CRT in μm at 4 wks |
|---|---|---|---|---|---|
| Bashshur et al 2006 | 17 | 1.0 | 0.6
(p = 0.001) |
350 | 282
(p < 0.001) |
| Abrahám-Marin et al 2006 | 39 | 1.18 | 0.88
(p = 0.002) |
388 | 247
(p < 0.001) |
Abbreviations: ARMD, age-related macular degeneration; BCVA, best corrected visual acuity; CRT, central retinal thickness.
Single and multiple 1.25 mg bevacizumab injections
Table 2 portrays the changes in BCVA after mostly single intravitreal injections of 1.25 mg bevacizumab in 35 patients. It is interesting to note that in the study by Moschos et al (2007) both CRT and BCVA showed a significant improvement at 4 weeks which did not last until 12 weeks, whereas the patients studied by Aggio et al (2006) still had significantly improved CRT and BCVA more than 12 weeks after injection.
Table 2.
Effect of mostly single intravitreal injections of 1.25 mg bevacizumab on mean logMAR BCVA and mean CRT (μm) in neovascular ARMD
| Authors | n = | Baseline CRT | Baseline BCVA | ↓CRT 4 wks | BCVA 4 wks | ↓CRT 12 wks | BCVA 12 wks | ↓CRT >12 wks | BCVA >12 wks |
|---|---|---|---|---|---|---|---|---|---|
| Moschos et al 2007 | 18 | 298.5 | 0.62 | 52.72
(p < 0.001) |
0.57
(p < 0.05) |
43.5
(p = 0.0592) |
0.60
(p = 0.2261) |
– | – |
| Aggio et al 2006 | 17 | 404.05 | 1.17 | – | – | – | – | 123.82
(p = 0.032) |
1.06
(p = 0.17) |
Abbreviations: ARMD, age-related macular degeneration; BCVA, best corrected visual acuity; CRT, central retinal thickness.
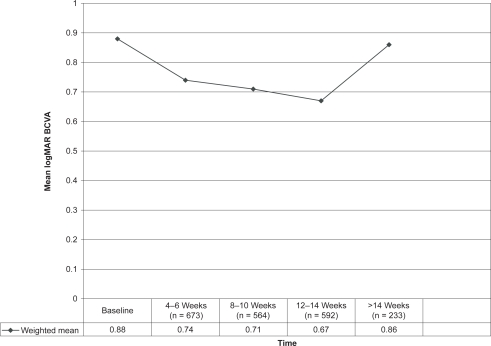
Table 3 summarizes the effect of repeated intravitreal injections of 1.25 mg bevacizumab on mean logMAR BCVA in 10 different studies. Weighted means were calculated to quantify the overall effect of the intervention and it was found that, compared with baseline, the mean BCVA had improved at 4–6 weeks, 8–10 weeks, and 12–14 weeks with the overall BCVA almost returning to the baseline value after 14 weeks even though some of the individual studies still showed significant improvement at this time. These findings are depicted in Figure 1.
Table 3.
Effect of repeated intravitreal injection of 1.25 mg bevacizumab on mean logMAR BCVA in patients with neovascular ARMD
| Authors | n = | Previous Rx (%) | Baseline | 4–6 wks | 8–10 wks | 12–14 wks | >14 wks |
|---|---|---|---|---|---|---|---|
| Avery et al 2006 | 81 | 63 (78) | 1.00 | 0.80 (p < 0.0001) | 0.80 (p < 0.0001) | – | – |
| Yoganathan et al 2006 | 50 | 36 (72) | 0.96 | 0.83 (p < 0.01) | 0.73 | 0.80 | 0.86 (P < 0.01) |
| Rich et al 2006 | 53 | 40 (75) | 0.94 | 0.82 (p < 0.001) | 0.76 (p = 0.001) | 0.78 (p < 0.001) | – |
| Spaide et al 2006 | 251 | 175 (69) | 0.96 | 0.84 (p < 0.001) | 0.79 (p < 0.001) | 0.74 (p < 0.001) | – |
| Lazic et al 2007 | 102 | 0 (0) | 1.07 | – | – | – | 0.94 (P = 0.001) |
| Aisenbrey et al 2006 | 30 | 4 (13) | 0.78 | 0.59 | – | 0.63 (p < 0.001) | – |
| Chen et al 2007 | 102 | 56 (55) | 0.60 | 0.50 (p < 0.001) | 0.40 (p < 0.001) | 0.40 (p < 0.05) | – |
| Giansanti et al 2007 | 27 | 14 (52) | 0.72 | 0.74 | 0.80 | 0.68 (P = 0.40) | 0.86 |
| Emerson et al 2007 | 79 | 47 (59) | 0.71 | 0.58 (p = 0.00037) | – | 0.63 (p = 0.04) | – |
| Goff et al 2007 | 54 | 38 (70) | 0.80 | – | – | – | 0.7 (p = 0.03) |
| Weighted mean | 829 | 0.88 (n = 829) | 0.74 (n = 673) | 0.71 (n = 564) | 0.67 (n = 592) | 0.86 (n = 233) |
Abbreviations: ARMD, age-related macular degeneration; BCVA, best corrected visual acuity; CRT, central retinal thickness.
Figure 1.
Effect of repeated intravitreal injections of 1.25 mg bevacizumab on mean logMAR best corrected visual acuity (BCVA).
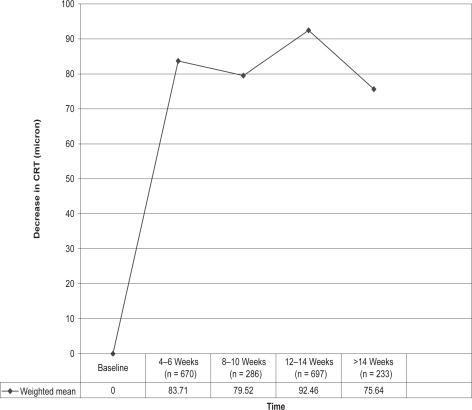
Table 4 shows the decrease in CRT recorded in the same 10 studies referred to above. Weighted means were again calculated and showed that at all time points the mean CRT was still well below that at baseline as seen in Figure 2. Most of the individual studies providing data after 14 weeks show that significant reductions in CRT can be maintained for more than 3 months with repeated IVB injections, although collectively the greatest reduction occurred between 12 and 14 weeks.
Table 4.
Decrease (Δ) in mean CRT (μm) after repeated intravitreal injections of 1.25 mg bevacizumab in patients with neovascular ARMD
| Authors | n = | Previous Rx (%) | Baseline CRT | 4–6 wks | 8–10 wks | 12–14 wks | >14 wks |
|---|---|---|---|---|---|---|---|
| Avery et al 2006 | 81 | 63 (78) | – | 92 (p < 0.0001) | 89 (P < 0.0001) | 67 (P < 0.01) | – |
| Yoganathan et al 2006 | 50 | 36 (72) | 339 | 70 (p < 0.001) | 99 | 63 | 73 (p < 0.001) |
| Rich et al 2006 | 53 | 40 (75) | 351 | 97.5 (p < 0.001) | 110.8 (P < 0.001) | 99.6 (P < 0.001) | – |
| Spaide et al 2006 | 251 | 175 (69) | 340 | 93 (p < 0.001) | – | 127 (P < 0.001) | – |
| Lazic et al 2007 | 102 | 0 (0) | 378 | – | – | – | 56 (p = 0.01) |
| Aisenbrey et al 2006 | 30 | 4 (13) | – | – | – | – | – |
| Chen et al 2007 | 102 | 56 (55) | 251 | 36.1 (p < 0.001) | 46.2 (P < 0.001) | 41 (P < 0.05) | – |
| Giansanti et al 2007 | 27 | 14 (52) | 373 | – | – | 94 (P < 0.01) | 70 (p < 0.01) |
| Emerson et al 2007 | 79 | 47 (59) | 304 | 77 (p < 0.00001) | – | 67 (P = 0.00002) | – |
| Goff et al 2007 | 54 | 38 (70) | 362 | 127 (p < 0.0001) | – | 124 (P = 0.0004) | 118 (P < 0.0001) |
| Weighted mean | 829 | 83.71 (n = 670) | 79.52 (n = 286) | 92.46 (n = 697) | 75.64 (n = 233) |
Abbreviations: ARMD, age-related macular degeneration; CRT, central retinal thickness.
Figure 2.
Decrease (Δ) in central retinal thickness (CRT) (μm) after repeated 1.25 mg bevacizumab injections in patients with neovascular age-related macular degeneration.
When comparing Figures 1 and 2 it will be noted that between 12 and 14 weeks the greatest reduction in CRT coincided with the greatest improvement in BCVA even though in most of the individual studies the correlation between BCVA and decrease in CRT was not statistically significant.
Diabetic retinopathy
It is well known that VEGF plays an important role in diabetic retinopathy and several reports of a beneficial effect on neovascularization in diabetic patients after IVB have been published.
In one case series (Mason et al 2006), 3 patients with proliferative diabetic retinopathy (PDR) received IVB and in all 3 cases complete regression of both neovascularization at the disc (NVD) and neovascularization elsewhere (NVE) was observed between 1 and 3 weeks post-injection. In 3 other case studies, NVD and/or NVE regressed completely within 7 (Avery 2006), 8 (Friedlander and Welch 2006), and 30 (Isaacs and Barry 2006) days, respectively. Angiographic leakage resolved completely in 73% of eyes with NVD and 82% of eyes with NVI after a single dose of IVB (Avery et al 2006) while in another study the mean area of actively leaking neovascularization decreased from 27.79 ± 6.29 mm2 to 5.43 ± 2.18 mm2 at 1 week and 5.50 ± 1.24 mm2 at 12 weeks post-injection (Jorge et al 2006). A third study reporting on 78 eyes of 64 patients with a minimum follow-up of 6 months showed a decrease in mean central macular thickness from 387.0 ± 182.8 μm at baseline to 275.7 ± 108.3 μm at the end of follow-up (p < 0.0001). In this report, 20.5% of eyes needed a second injection and 7.7% needed a third (Arevalo et al 2007). All three studies demonstrated an improvement in BCVA over the treatment period (Table 5).
Table 5.
Effect of intravitreal bevacizumab on BCVA in patients with diabetic retinopathy
| Authors | n = | Dose | Mean follow-up | Mean logMAR BCVA at 0 wks | Mean logMAR BCVA at end of study |
|---|---|---|---|---|---|
| Avery et al 2006 | 45 | 6.2 μg–1.25 mg | 5 wks | 1.00 | 0.796 |
| Jorge et al 2006 | 15 | 1.5 mg | 12 wks | 0.90 | 0.77 |
| Arevalo et al 2005 | 78 | 1.25 mg or 2.5 mg | 6.31 ± 0.81 months | 0.87 | 0.6 |
| Weighted mean | 138 | 0.92 | 0.68 |
Abbreviations: BCVA, best corrected visual acuity.
IVB has been used successfully in the treatment of neovascularization of the iris (NVI) in instances where both panretinal photocoagulation (PRP) and pars plana vitrectomy (PPV) have failed to halt the progression of NVI (Oshima et al 2006). It has also been used to good effect in patients with recurrent vitreous hemorrhage despite complete PRP (Bakri et al 2006) as well as vitreous hemorrhage precluding PRP (Spaide and Fisher 2006). Preoperative IVB in a patient with a pre-retinal neovascular membrane and underlying tractional retinal detachment induced significant regression of the neovascular component and thereby facilitated removal of the fibrous membrane with minimal intra-operative hemorrhage (Chen and Park 2006).
Fifty-one patients with persistent diffuse diabetic macular edema despite previous treatments such as focal or panretinal laser therapy, vitrectomy, and intravitreal triamcinolone injection were treated with IVB. Their mean logMAR VA improved from 0.86 ± 0.38 at baseline to 0.75 ± 0.37 and 0.84 ± 0.41 at 6 and 12 weeks respectively while their mean central retinal thickness decreased from 501 ±163 μm at baseline to 416 ± 180 μm at 6 weeks and 377 ± 117 μm at 12 weeks (Haritoglou et al 2006).
Neovascular glaucoma
IVB has been used to address neovascularization in neovascular glaucoma (NVG) secondary to proliferative diabetic retinopathy (PDR), central retinal vein occlusion (CRVO) and central retinal artery occlusion (CRAO) (Mason et al 2006; Vatavuk et al 2007).
A patient with NVG secondary to PDR underwent IVB injection since PRP was precluded by vitreous hemorrhage (Paula et al 2006). After 1 day the eye was pain free and both NVI and neovascularization of the angle (NVA) showed partial regression while after 1 week the NVI and NVA had regressed completely on iris fluorescein angiography. At 8 weeks the new vessels had not recurred and the IOP was 19 mm Hg on topical timolol and brimonidine as well as oral acetazolamide.
Six patients with refractory NVG after retinal vein occlusions received IVB since the mean IOP on maximal therapy was 36.0 ± 5.1 mmHg (Iliev et al 2006). PRP was performed as soon as possible thereafter and only 3 eyes needed further cyclodiode laser to control the IOP. At the end of the follow-up period the IOP was 16.8 ± 3.5 mmHg and all the patients were symptom-free. Another patient with similar pathology received IVB for ocular discomfort despite maximal tolerated therapy and undergoing both cyclodiode laser and PRP previously (Kahook et al 2006). After 48 hours her discomfort had resolved and IOP had decreased from 48 to 22 mmHg. She was then able to receive further PRP and decrease her topical medication.
Pathologic myopia (PM)
After publication of reports about the beneficial effect of IVB in neovascular ARMD, various centers offered this treatment to patients with CNV secondary to pathologic myopia (mCNV). Many of these patients had already undergone one or more treatments with PDT without much success.
In one study, 8 eyes of 8 patients with mCNV were treated with IVB (Sakaguchi et al 2006). Angiographic leakage from the CNV was reduced in 87.5% of eyes with mean decimal Snellen BCVA improving from 0.26 to 0.51, mean CNV size decreasing from 0.72 ± 0.78 disc areas (DA) to 0.64 ± 0.73 DA, and mean foveal thickness decreasing from 198.4 ± 66.5 μm to 155.1 ± 74.6 μm. Eleven eyes of 9 patients received IVB in another small study (Yamamoto et al 2006) where 5 of the eyes had previously been treated with PDT. Mean VA improved by +3.5 lines and mean central foveal thickness improved from 340 μm to 234 μm.
One patient (Tewari et al 2006), whose VA remained 20/200 6 weeks after PDT, received 2 doses of IVB 6 months apart and on final examination had a VA of 20/25 and no sub-retinal fluid on OCT examination. Similar results are also reported in another case series (Laud et al 2006).
Retinal vein occlusion
Macular edema resulting from central retinal vein occlusion (CRVO) often responds favorably to intravitreal injection of triamcinolone acetonide (Greenberg et al 2002; Park et al 2003). IVB has been used in instances where triamcinolone acetonide did not have the desired effect on macular edema or caused either progression of cataract or raised intraocular pressure.
Three separate studies examined the effect of 1.25 mg IVB injections on BCVA and CRT in patients with macular edema secondary to different types of retinal vein occlusion. The results are summarized in Tables 6 and 7.
Table 6.
Effect of intravitreal injection of bevacizumab on BCVA in RVO patients receiving a dose of 1.25 mg
| Authors | Type of RVO | N = | Baseline | 4 wks | 12 wks |
|---|---|---|---|---|---|
| Iturralde et al 2006 | CRVO | 16 | 1.48 | 1.05 (p = 0.001) | 0.84 (p < 0.001) |
| Pai et al 2007 | 9 CRVO and 12 BRVO | 21 | 1.28 | 0.83 (p = 0.001) | 0.95 (p = 0.001) |
| Rabena et al 2007 | BRVO | 27 | 1.0 | 0.69 | 0.70 |
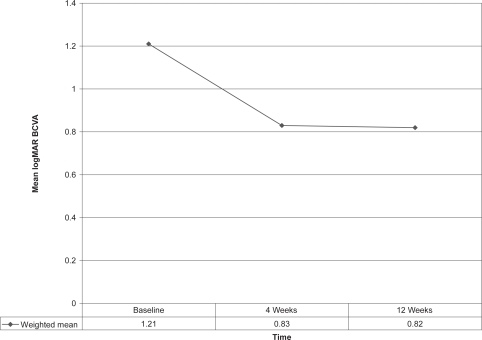
| Weighted mean | 64 | 1.21 | 0.83 | 0.82 |
Abbreviations: BCVA, best corrected visual acuity; RVO, retinal vein occlusion.
Table 7.
Effect of intravitreal injection of bevacizumab on CRT (μm) in RVO patients receiving a dose of 1.25 mg
| Authors | Type of RVO | N = | Baseline | 4 wks | 12 wks |
|---|---|---|---|---|---|
| Iturralde et al 2006 | CRVO | 16 | 887 | 372 (p < 0.001) | – |
| Pai et al | 9 CRVO and 12 BRVO | 21 | 647.81 | 293.43 (p = 0.001) | 320.90 (p = 0.001) |
| Rabena et al 2007 | BRVO | 27 | 478 | 310 | 366 |
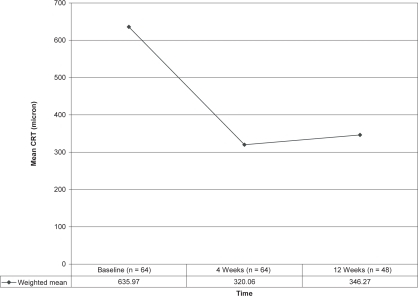
| Weighted mean | 635.97 (n = 64) | 320.06 (n = 64) | 346.27 (n = 48) |
Abbreviations: CRT, central retinal thickness; RVO, retinal vein occlusion.
After calculating weighted means, the BCVA showed marked improvement at 4 weeks and remained stable at 12 weeks whereas CRT decreased significantly after 4 weeks but then showed a small increase by 12 weeks. These findings are depicted in Figures 3 and 4. Similar favorable results were noted in other single case reports (Rosenfeld et al 2005; Spandau et al 2006).
Figure 3.
Effect of 1.25 mg intravitreal bevacizumab injection on mean logMAR best corrected visual acuity (BCVA) in retinal vein occlusion patients (n = 64).
Figure 4.
Effect of 1.25 mg intravitreal bevacizumab injection on mean central retinal thickness CRT (μm) in retinal vein occlusion patients.
Seven patients with macular edema secondary to ischemic central or hemicentral retinal vein occlusion (RVO) received intravitreal injections of 2.0 mg bevacizumab at 12-week intervals (Costa et al 2007). Mean logMAR BCVA improved from 1.21 at baseline to 0.68 at 25-week follow-up while CRT and total macular volume respectively improved from 730.1 μm and 17.1 mm3 at baseline to 260.3 μm and 9.0 mm3 at 25 weeks.
One patient with a BRVO and 2 patients with CRVO were treated with IVB but, after an initial favorable response, developed rebound macular edema in excess of what was noted before the first intravitreal injection (Matsumoto et al 2007).
In an interesting case where a patient developed bilateral combined central retinal artery occlusion and central retinal vein occlusion as a result of thrombotic thrombocytopenic purpura secondary to adult-onset Still’s disease, IVB combined with PRP brought about resolution of iris neovascularization and thus precluded the need for cyclo-photoablation (Schwartz et al 2006).
Other reported uses of intravitreal bevacizumab
The use of IVB has been attempted with varying degrees of success in a variety of retinal conditions other than those already discussed (Table 8).
Table 8.
Other reported uses of intravitreal bevacizumab
|
Abbreviation: CNV, choroidal neovascularization.
A patient with subfoveal CNV related to angioid streaks and VA of counting fingers at 2 metres was treated with 2 injections of IVB 7 weeks apart. Eighteen weeks later the VA in the affected eye was 20/30 (Teixeira et al 2006). IVB was also used in a patient with serous detachment of the macula and extensive sub-retinal hemorrhage from peripapillary CNV. Within 3 months the serous detachment had completely resolved and the patient’s BCVA had improved from 0.16 decimal Snellen to 1.0 (Soliman et al 2006). Similarly, VA improved from 20/40−1 to 20/20 over a 24-week period in a patient with idiopathic juxtafoveal retinal telangiectasis and juxtafoveal subretinal neovascularization (Jorge et al 2006).
Another patient with retinal neovascularization and vitreous hemorrhage secondary to sickle cell retinopathy received IVB after declining vitrectomy with endophotocoagulation. Four weeks later, the VA had improved from 20/60 to 20/20, the neovascularization had regressed and scatter laser photocoagulation could be applied (Siqueira et al 2006). Two patients with pseudophakic cystoid macular edema refractory to conventional medical treatment received IVB which resulted in a marked VA improvement in both cases (Mason et al 2006). IVB also caused regression of iris, retinal, and choroidal neovascularization in a case of anterior segment ischemia caused by laser ablation for aggressive posterior retinopathy of prematurity (Shah et al 2007). IVB was attempted in 6 patients with chronic macular edema due to uveitis but, although well tolerated, none of the patients had significant improvement in either visual acuity or central retinal thickness after 1 month (Ziemssen et al 2007).
Nine patients with idiopathic CNV, 2 with CNV secondary to central serous chorioretinopathy (CSCR), and 4 with CNV attributed to punctuate inner choroidopathy (PIC) received monthly 1.25 mg IVB injections for 3 months (Chan et al 2007). Mean logMAR BCVA improved from 0.48 at baseline to 0.25 at 1 month and 0.17 at 6 months (both p = 0.001) while mean CRT declined from 306 μm at baseline to 201 μm at 6 months (p < 0.001). No recurrences were observed at 6 months. Both BCVA and CRT also improved significantly after IVB in 10 eyes with idiopathic CNV refractory to posterior subtenon triamcinolone injection for more than 3 months (Gomi et al 2007).
Twenty-three patients with retinal angiomatous proliferation (RAP) were treated with 1.25 mg IVB during a 3-month period (Meyerle et al 2007). Median logMAR visual acuity improved from 0.60 at baseline to 0.48 at both 1 and 3 months. Mean CRT improved from 335 μm at baseline to 202 μm at 1 month and 209 μm at 3 months. Nine eyes had pigment epithelial detachments (PEDs) at baseline and only 2 PEDs were present at 1 month. Similar results were reported in 16 other patients with RAP (Joeres et al 2007).
Discussion
Several chemical mediators have been implicated in the regulation of intraocular neovascularization. These include angiopoietin (Otani et al 1999), pigment epithelium-derived factor (PEDF) (Holekamp et al 2002), fibroblast growth factor (Schlingemann 2004), and VEGF.
VEGF promotes the proliferation and migration of vascular endothelial cells and organizes these cells into functioning tubules. It increases vascular permeability and is involved in vessel maturation and regression as well as ocular inflammation (Ferrara 2004; Adamis and Shima 2005). As such, it is believed to be a key factor in the development and progression of both CNV (Ishibashi et al 1997) and ischemia-induced retinal neovascularization (Ishida et al 2003).
Intravitreal injection of VEGF into normal primate eyes causes microvascular occlusion with ischemia, telangiectasis, and even micro-aneurysm formation – all features of diabetic retinopathy (Tolentino et al 1996, 2002). Not only do VEGF levels show a correlation with the severity of proliferative diabetic retinopathy but also a reduction in levels once the retinopathy has been successfully treated with laser photocoagulation (Aiello et al 1994). VEGF injection can induce iris neovascularization and neovascular glaucoma (Tolentino et al 1996) and it plays an important role in the pathogenesis of diabetic macular edema by increasing vascular permeability (Funatsu et al 2006).
Over the past few decades various modalities have become available for the treatment of neovascular ocular diseases but all of these have had limitations. Photodynamic therapy (PDT) with verteporfin (Novartis Ophthalmics, Duluth, GA, USA), alone or in combination with intravitreal injection of triamcinolone acetonide, reduces the risk of vision loss in patients suffering from exudative ARMD but, despite this reduction in risk, the mean visual acuity decreases over time (TAP 1999; VIP 2001). PDT is also approved for treatment of subfoveal CNV in pathologic myopia (PM) but in one study, despite showing benefit at 1 year, no statistically significant treatment benefit was demonstrated at 2 years (VIP 2003).
Laser thermal photocoagulation is used in a variety of retinal neovascular diseases (MPSG 1991) but, due to photoreceptor damage, is no longer indicated for the treatment of subfoveal CNV in conditions such as ARMD and PM. Indocyanine green-mediated photothrombosis (Arevalo et al 2005) and transpupillary thermotherapy (Connoly et al 2005) have also been described in the treatment of CNV.
The idea of inhibiting or counteracting angiogenic factors is not new. In 1948, Michaelson proposed the role of growth factors in the development of vascular diseases of the retina (Michaelson 1948) and in 1971 Folkman suggested that inhibiting these factors may have therapeutic implications in the treatment of cancer (Folkman 1971).
Pegaptanib sodium (Macugen®; Eyetech Pharmaceuticals, New York, NY) is an aptamer that binds VEGF isomer 165 (Gragoudas et al 2004) and was the first anti-VEGF drug to be approved by the FDA in December 2004 for intravitreal injection in the treatment of CNV. Patients treated with repeated intravitreal injections of pegaptanib experienced a slower rate of vision loss when compared with a control group after 1 year although only 6% had a significant improvement in vision as compared with 2% in the control group.
Ranibizumab (Lucentis®; Genentech, South San Francisco, CA) is an antigen binding fragment (Fab) developed from a full-length recombinant humanized monoclonal antibody with the purpose of improving retinal penetration due to its smaller size. Unlike pegaptanib it binds to all isoforms of VEGF. In a recent study, patients were treated with 24 monthly intravitreal injections of 0.3 or 0.5 mg ranibizumab. At 12 months, 94.5% (0.3 mg) and 94.6% (0.5 mg), respectively, lost fewer than 15 letters as opposed to 62.2% in the control group. Of note, visual acuity improved by ≥15 letters in 24.8% (0.3 mg) and 33.8% (0.5 mg) compared with 5.0% in the control group (Rosenfeld et al 2006). Ranibizumab received FDA approval for intravitreal use on 30 June 2006.
Bevacizumab (Avastin®; Genentech, South San Francisco, CA) is the full-length monoclonal antibody that was initially considered to be too large to penetrate the retina and thus led to the development of its derivative ranibizumab. It also binds all isomers of VEGF (Ferrara et al 2004) but, unlike ranibizumab with a single VEGF binding site, it has two VEGF binding sites that increase its overall dissociation constant for VEGF to between 0.5 and 1.0 nM compared with 0.140 nM for ranibizumab (Chen et al 1999).
In the literature under review, intravitreal injection of bevacizumab showed a definite improvement in both BCVA and central retinal thickness in a variety of neovascular conditions affecting the eye. In neovascular ARMD it not only improved these parameters in the 53% of patients who had already received more conventional forms of treatment but also in the 47% who received IVB as primary therapy. One study even suggested that patients receiving IVB as primary treatment fared better than those who had previously undergone other treatment. The literature shows that the beneficial effect of a single IVB injection lasts up to 12 weeks but also that this effect is prolonged by repeated injections of the drug.
IVB appears useful in addition to different forms of laser therapy in both diabetic retinopathy and neovascular glaucoma. It not only causes regression of new vessels when conventional photocoagulation appears to have been unsuccessful but can also keep neovascularization in check in patients with vitreous hemorrhage until such time as the view of the fundus has improved and laser photocoagulation can be applied. IVB may facilitate the use of focal laser in exudative diabetic maculopathy by decreasing macular swelling and thereby improving uptake of the laser burns. The report (Chen and Park 2006) that preoperative IVB reduces the neovascular component of fibrovascular membranes in advanced diabetic retinopathy and therefore makes the surgical removal technically easier will probably be of interest to retinal surgeons worldwide. The rapid resolution of pain and possible preclusion of diode laser cycloablation and systemic acetazolamide from the treatment of neovascular glaucoma are exciting prospects in a condition that can prove difficult to manage.
In pathologic myopia, it is interesting to note that IVB had a beneficial effect on BCVA, mean CNV size and central retinal thickness even in cases where multiple treatments with PDT were not successful (Sakaguchi et al 2006). This brings hope in a condition that until now has caused severe visual loss with a poor prognosis in young and middle-aged patients. IVB, like intravitreal triamcinolone, improves BCVA and macular swelling in patients with RVO. The obvious advantage of IVB is that, unlike triamcinolone, it has thus far not been shown to increase intraocular pressure or expedite cataract formation.
Apart from its apparent efficacy, IVB also appears to be relatively safe in the short-term with no cases of ocular toxicity, retinal detachment, raised intraocular pressure, or thromboembolic events being reported in any of the literature reviewed. However, 2 cases of acute endophthalmitis occurred 2 days after IVB and coagulase-negative staphylococci were isolated from vitreous specimens in both cases (Aggio et al 2007) while two potentially drug-related adverse events (ischemic stroke and myocardial infarction) where reported elsewhere. One study reported a 20.5% incidence of mild anterior chamber inflammation not requiring any treatment while another reported 2 out of 224 patients (0.8%) with mild vitritis 1 month after injection. Another study reporting side effects other than commonly encountered subconjunctival hemorrhage and local conjunctival hyperemia after injection noted 2 retinal pigment epithelium rips and 10 posterior vitreous detachments in 102 patients, while several other authors have also encountered retinal pigment epithelium tears after injecting IVB for neovascular AMD (Meyer et al 2006; Nicolo et al 2006; Shah et al 2006). A case of mild anterior uveitis that responded well to topical steroids has also been reported (Pieramici et al 2006).
As alluded to earlier, there are several limitations in compiling a review such as this one. Inconsistency in both the methods of acquisition and reporting of data, lack of standardized treatment protocols, variable timing of follow-up visits, the retrospective nature of most reports, lack of controls, and relatively high percentage of patients lost to follow-up all play a role in this regard. However, despite these limitations it is still possible to gain a better overall impression of the effect of IVB in several different ocular diseases and begin to quantify its effect.
Conclusion
It appears as if IVB, despite its off-label use, is both efficacious and relatively safe in the short-term when used for a variety of neovascular ocular conditions and at present is a useful adjunct to conventional treatment. Potential advantages include the fact that it is much cheaper than the other VEGF inhibitors already approved for intravitreal use and, in animal studies, has a longer half-life in the vitreous than, for example, ranibizumab. Potential disadvantages include the still relatively short half-life which necessitates frequent re-injection to maintain the beneficial effect and subsequent exposure to the risks that accompany any intravitreal injection.
The results reviewed in this article need to be confirmed by large prospective, randomized trials using carefully standardised protocols with regard to inclusion/exclusion criteria, dosages injected, criteria for and timing of re-injection, follow-up intervals, and duration of treatment in an attempt to better clarify the exact indications for IVB as well as duration of beneficial effect and optimal dose of IVB. There can, however, be little doubt that once longer-acting VEGF-inhibitors become available that can possibly be administered by less invasive routes the need for destructive laser procedures and even posterior vitrectomy will diminish.
References
- Abraham-Marin ML, Cortes-Luna CF, Alvarez-Rivera G, et al. 2006Intravitreal bevacizumab therapy for neovascular age-related macular degeneration: a pilot study Graefes Arch Clin Exp Ophthalmol September28[Epub ahead of print] [DOI] [PubMed]
- Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularisation in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–8. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Aggio FB, Farah ME, de Melo GB, et al. Acute endophthalmitis following intravitreal bevacizumab (Avastin) injection. Eye. 2007;21:408–9. doi: 10.1038/sj.eye.6702683. [DOI] [PubMed] [Google Scholar]
- Aggio FB, Farah ME, Silva WC, et al. 2006Intravitreal bevacizumab for exudative age-related macular degeneration after multiple treatments Graefes Arch Clin Exp Ophthalmol December1[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aisenbrey S, Ziemssen F, Volker M, et al. 2006Intravitreal bevacizumab (Avastin) for occult neovascularisation in age-related macular degeneration Graefes Arch Clin Exp Ophthalmol December21[Epub ahead of print] [DOI] [PubMed]
- Arevalo JF, Carcia RA, Mendoza AJ. Indocyanine green-mediated photothrombosis with intravitreal triamcinolone acetonide for subfoveal choroidal neovascularisation in age-related macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2005;243:1180–5. doi: 10.1007/s00417-005-1177-y. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Avery RL. Regression of retinal and iris neovascularisation after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Donaldson MJ, Link TP. Rapid regression of disc neovascularisation in a patient with proliferative diabetic retinopathy following adjunctive intravitreal bevacizumab. Eye. 2006;20:1474–5. doi: 10.1038/sj.eye.6702364. [DOI] [PubMed] [Google Scholar]
- Bashshur ZF, Bazarbachi A, Schakal A, et al. Intravitreal bevacizumab for the management of choroidal neovascularisation in age-related macular degeneration. Am J Ophthal. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Liu DT, et al. 2007Intravitreal bevacizumab (Avastin) for choroidal neovascularisation secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy or of idiopathic origin Am J Ophthalmol April23[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen CY, Wong TY, Heriot WJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration: a short-term study. Am J Ophthalmol. 2007;143:510–2. doi: 10.1016/j.ajo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699–700. doi: 10.1097/01.iae.0000225351.87205.69. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–81. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- Connoly BP, Regillo CD, Eagle RC, Jr, et al. The histopathologic effects of transpupillary thermotherapy in human eyes. Ophthalmology. 2003;110:415–20. doi: 10.1016/S0161-6420(02)01561-0. [DOI] [PubMed] [Google Scholar]
- Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularisation caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (Avastin) for central and hemicentral retinal vein occlusions: IBeVO Study. Retina. 2007;27:141–9. doi: 10.1097/IAE.0b013e31802eff83. [DOI] [PubMed] [Google Scholar]
- Cotter SA, Chu RH, Chandler DL, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003;136:655–61. doi: 10.1016/s0002-9394(03)00388-x. [DOI] [PubMed] [Google Scholar]
- Emerson MV, Lauer AK, Flaxel CJ, et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007;27:439–44. doi: 10.1097/IAE.0b013e31804b3e15. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Finger PT. Anti-VEGF bevacizumab (Avastin) for radiation optic neuropathy. Am J Ophthalmol. 2007;143:335–8. doi: 10.1016/j.ajo.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Friedlander SM, Welch RM. Vanishing disc neovascularisation following intravitreal bevacizumab (Avastin) injection. Arch Ophthalmol. 2006;124:1365. doi: 10.1001/archopht.124.9.1365. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular oedema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Giansanti F, Virgili G, Bini A, et al. Intravitreal bevacizumab therapy for choroidal neovascularisation secondary to age-related macular degeneration: 6-month results of an open-label uncontrolled clinical study. Eur J Ophthalmol. 2007;17:230–7. doi: 10.1177/112067210701700213. [DOI] [PubMed] [Google Scholar]
- Goff MJ, Johnson RN, McDonald HR, et al. Intravitreal bevacizumab for previously treated choroidal neovascularisation from age-related macular degeneration. Retina. 27:432–8. doi: 10.1097/IAE.0b013e318042b53f. [DOI] [PubMed] [Google Scholar]
- Gomi F, Nishida K, Oshima Y, et al. Intravitreal bevacizumab for idiopathic choroidal neovascularisation after previous injection with posterior subtenon triamcinolone. Am J Ophthalmol. 2007;143:507–10. doi: 10.1016/j.ajo.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular edema due to central retinal vein occlusion. Br J Ophthalmol. 2002;86:247–8. doi: 10.1136/bjo.86.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularisation due to age-related macular degeneration. Am J Ophthalmol. 2002;134:220–7. doi: 10.1016/s0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–6. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Isaacs TW, Barry C. Rapid resolution of severe disc new vessels in proliferative diabetic retinopathy following a single intravitreal injection of bevacizumab (Avastin) Clin Experiment Ophthalmol. 2006;34:802–3. doi: 10.1111/j.1442-9071.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Hata Y, Yoshikawa H, et al. Expression of vascular endothelial growth factor in experimental choroidal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 1997;235:159–67. doi: 10.1007/BF00941723. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, et al. VEGF 164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularisation. J Exp Med. 2003;198:483–9. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Joeres S, Heussen FM, Treziak T, et al. 2007Bevacizumab (Avastin) treatment in patients with retinal angiomatous proliferation Graefes Arch Clin Exp Ophthalmol April17[Epub ahead of print] [DOI] [PubMed]
- Jonas JB, Spandau UH, Schlichtenbrede F. Intravitreal bevacizumab for filtering surgery. Ophthalmic Res. 2007;39:121–2. doi: 10.1159/000099248. [DOI] [PubMed] [Google Scholar]
- Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26:1006–13. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) associated with the regression of subretinal neovascularisation in idiopathic juxtafoveolar retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol. 2006. Nov 29, [Epub ahead of print] [DOI] [PubMed]
- Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144–6. [PubMed] [Google Scholar]
- Kumar A, Sinha S. Rapid regression of disc and retinal neovascularisation in a case of Eales disease after intravitreal bevacizumab. Can J Ophthalmol. 2007;42:335–6. [PubMed] [Google Scholar]
- Laud K, Spaide RF, Freund KB, et al. Treatment of choroidal neovascularisation in pathologic myopia with intravitreal bevacizumab. Retina. 2006;26:960–3. doi: 10.1097/01.iae.0000240121.28034.c3. [DOI] [PubMed] [Google Scholar]
- Lazic R, Gabric N. Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularisation secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:68–73. doi: 10.1007/s00417-006-0466-4. [DOI] [PubMed] [Google Scholar]
- [MPSG] Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–14. [PubMed] [Google Scholar]
- Mason JO, 3rd, Albert MA, Jr, Mays A, et al. Regression of neovascular iris vessels by intravitreal injection of bevacizumab. Retina. 2006;26 doi: 10.1097/01.iae.0000230425.31296.3b. [DOI] [PubMed] [Google Scholar]
- Mason JO, 3rd, Albert MA, Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic macular edema. Retina. 2006;26:356–7. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- Mason JO, 3rd, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:685–8. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Freund KB, Peiretti E, et al. Rebound macular edema following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007;27:426–31. doi: 10.1097/IAE.0b013e31804a7af2. [DOI] [PubMed] [Google Scholar]
- Melo GB, Farah ME, Aggio FB. Intravitreal injection of bevacizumab for cystoid macular edema in retinitis pigmentosa. Acta Ophthalmol Scand. 2007. Feb 19, [Epub ahead of print] [DOI] [PubMed]
- Meyer CH, Mennel S, Schmidt JC, et al. Acute retinal epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularisation secondary to age related macular degeneration. Br J Ophthalmol. 2006;90:1207–8. doi: 10.1136/bjo.2006.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerle CB, Freund KB, Iturralde D, et al. Intravitreal bevacizumab (Avastin) for retinal angiomatous proliferation. Retina. 2007;27:451–7. doi: 10.1097/IAE.0b013e318030ea80. [DOI] [PubMed] [Google Scholar]
- Michaelson IC. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68 [Google Scholar]
- Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Berger AS, Tolentino MJ. Intravitreal bevacizumab for macular edema from idiopathic juxtafoveal retinal telangiectasis. Ophthalmic Surg Lasers Imaging. 2007;38:164–6. doi: 10.3928/15428877-20070301-15. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Brouzas E, Apostolopoulos M, et al. Intravitreal use of bevacizumab (Avastin) for choroidal neovascularisation due to ARMD: a preliminary multifocal-ERG and OCT study: Multifocal ERG after use of bevacizumab in ARMD. Doc Ophthalmol. 2007;114:37–44. doi: 10.1007/s10633-006-9036-7. [DOI] [PubMed] [Google Scholar]
- Moshfeghi AA, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twenty four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002–11. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Shah S, Tatlipinar S, et al. Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia (letter) Br J Ophthalmol. 2005;89:1368–70. [PMC free article] [PubMed] [Google Scholar]
- Nicolo M, Ghiglione D, Calabria G. Retinal pigment epithelial tear following intravitreal injection of bevacizumab (Avastin) Eur J Ophthalmol. 2006;16:770–3. doi: 10.1177/112067210601600521. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sakaguchi H, Gomi F, et al. Regression of iris neovascularisation after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:155–8. doi: 10.1016/j.ajo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Otani A, Takagi H, Oh H, et al. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1912–20. [PubMed] [Google Scholar]
- Pai SA, Shetty R, Vijayan PB, et al. Clinical, anatomic and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol. 2007;143:601–6. doi: 10.1016/j.ajo.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136 doi: 10.1016/s0002-9394(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Paula JS, Jorge R, Costa RA, et al. Short-term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularisation in neovascular glaucoma. Acta Ophthalmol Scand. 2006;84:556–7. doi: 10.1111/j.1600-0420.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- Pieramici DJ, Avery RL, Castellarin AA, et al. Case of anterior uveitis after intravitreal injection of bevacizumab. Retina. 2006;26:841–2. doi: 10.1097/01.iae.0000234629.22614.cd. [DOI] [PubMed] [Google Scholar]
- Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007;27:419–25. doi: 10.1097/IAE.0b013e318030e77e. [DOI] [PubMed] [Google Scholar]
- Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26:495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol. 2006. Aug 16, [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- Schwartz SG, Hickey M, Puliafito CA. Bilateral CRAO and CRVO from thrombotic thrombocytopenic purpura: OCT findings and treatment with triamcinolone acetonide and bevacizumab. Ophthalmic Surg Lasers Imaging. 2006;37:420–2. doi: 10.3928/15428877-20060901-10. [DOI] [PubMed] [Google Scholar]
- Shah CP, Hsu J, Garg SJ, et al. Retinal pigment epithelium tear after intravitreal bevacizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2006;142:1068–70. doi: 10.1016/j.ajo.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Shah MA, Ilson D, Kelsen DP. Thrombo-embolic events in gastric cancer: high incidence in patients receiving irinotecan- and bevacizumab-based therapy. J Clin Oncol. 2005;23:2574–6. doi: 10.1200/JCO.2005.81.908. [DOI] [PubMed] [Google Scholar]
- Shah PK, Narendran V, Tawansy KA, et al. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischaemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–6. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- Siqueira RC, Costa RA, Scott IU, et al. Intravitreal bevacizumab (Avastin) injection associated with regression of retinal neovascularisation caused by sickle cell retinopathy. Acta Ophthalmol Scand. 2006;84:834–5. doi: 10.1111/j.1600-0420.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- Soliman W, Lund-Andersen H, Larsen M. Resolution of subretinal haemorrhage and fluid after intravitreal bevacizumab in aggressive peripapillary subretinal neovascularisation. Acta Ophthalmol Scand. 2006;84:707–8. doi: 10.1111/j.1600-0420.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous haemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment for choroidal neovascularisation secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- Spandau UH, Ihloff AK, Jonas JB. Intravitreal bevacizumab treatment of macular edema due to central retinal vein occlusion. Acta Ophthalmol Scand. 2006;84:555–6. doi: 10.1111/j.1600-0420.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- [TAP] Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin. One-year results of two randomized clinical trials – TAP report 1. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- Teixeira A, Moraes N, Farah ME, et al. Choroidal neovascularisation treated with intravitreal injection of bevacizumab (Avastin) in angioid streaks. Acta Ophthalmol Scand. 2006;84:835–6. doi: 10.1111/j.1600-0420.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- Tewari A, Dhalla MS, Apte RS. Intravitreal bevacizumab for treatment of choroidal neovascularisation in pathologic myopia. Retina. 2006;26:1093–4. doi: 10.1097/01.iae.0000254896.78766.74. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002;133:373–85. doi: 10.1016/s0002-9394(01)01381-2. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–8. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularisation and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114:964–70. doi: 10.1001/archopht.1996.01100140172010. [DOI] [PubMed] [Google Scholar]
- [VIP] Verteporfin in Photodynamic Therapy (VIP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – VIP report 2. Am J Ophthalmol. 2001;131:541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- [VIP] Verteporfin in Photodynamic Therapy (VIP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial – VIP report no 3. Ophthalmology. 2003;110:667–73. doi: 10.1016/s0161-6420(02)01998-x. [DOI] [PubMed] [Google Scholar]
- Vatavuk Z, Bencic G, Mandic Z. Intravitreal bevacizumab for neovascular glaucoma following central retinal artery occlusion. Eur J Ophthalmol. 2007;17:269–71. doi: 10.1177/112067210701700220. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathologic myopia. Br J Ophthalmol. 2006. Jul 26, [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Yoganathan P, Deramo VA, Lai JC, et al. Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006;26:994–8. doi: 10.1097/01.iae.0000244380.34082.67. [DOI] [PubMed] [Google Scholar]
- Ziemssen F, Deuter CM, Stuebiger N, et al. Weak transient response of chronic uveitic macular edema to intravitreal bevacizumab (Avastin) Graefes Arch Clin Exp Ophthalmol. 2007. Jan 12, [Epub ahead of print] [DOI] [PubMed]
- Ziemssen F, Voelker M, Altpeter E, et al. Intravitreal bevacizumab treatment of radiation maculopathy due to brachytherapy in choroidal melanoma. Acta Ophthalmol Scand. 2007. Feb 26, [Epub ahead of print] [DOI] [PubMed]