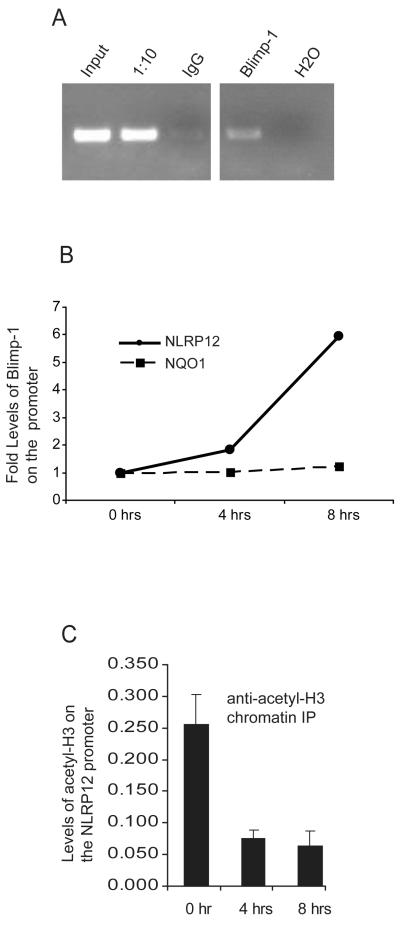
FIGURE 5.
Blimp-1 is recruited to the NLRP12 promoter after TLR stimulation in vivo. (A) Human U937 cells were stimulated with LPS for 8 hours followed by CHIP using a control IgG antibody, an antibody to acetylated Histone3, and an antibody to Blimp-1. DNA was eluted from the CHIP and subjected to PCR with primers specific for the NLRP12 promoter. PCR products were analyzed on an agarose gel. Lanes include PCR product obtained from input DNA, a 1/10 dilution of input DNA, immunoprecipitation with an IgG control antibody, immunoprecipitation with an anti-Blimp-1 antibody, and water as a PCR control. Data are representative of three separate experiments. (B) Chromatin Immunoprecipitation was performed to confirm the interaction of Blimp-1 with the NLRP12 promoter in vivo. The human monocyte cell line U937 was stimulated with LPS for 1, 4 and 8 hours. Chromatin bound Blimp-1 complexes were immunoprecipitated using an anti-Blimp-1 antibody followed by analysis of complexed chromatin by qPCR using primers specific for the NLRP12 promoter. qPCR using primers specific for the NQO1 promoter were used as a negative control. qPCR values have been normalized to input. Precipitation with an unrelated antibody was used as negative control. Data are a representative of three separate experiments. (C) Chromatin Immunoprecipitation was performed to measure acetylated-Histone 3 (H3) levels with the NLRP12 promoter in vivo. The human monocyte cell line U937 was stimulated with LPS for 1, 4 and 8 hours. Chromatin bound acetylated-H3 complexes were immunoprecipitated using an anti- acetylated-H3 antibody followed by analysis of complexed chromatin by qPCR using primers specific for the NLRP12 promoter. qPCR values have been normalized to input. Data are an average of four experiments.