Table 3.
Optimization Studies for the Asymmetric Reduction of Pyridyl Ethanone O-Benzyl Oximes
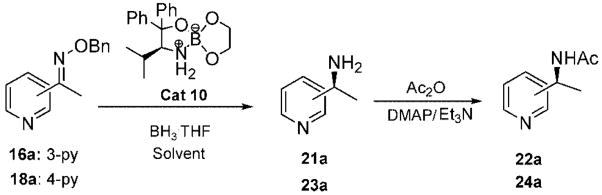 | |||||||
|---|---|---|---|---|---|---|---|
| entry | benzyl oxime | cat 10 (equiv) | T (°C) | solvent | time (h) | yield (%)a | ee (%)b |
| 1 | 16a | 0.1 | 0 | THF | 72 | 46 | 92 |
| 2 | 16a | 0.1 | 25 | THF | 48 | 53 | 89 |
| 3 | 16a | 0.1 | 0 | t-BuOMe | 72 | 42 | 93 |
| 4 | 16a | 0.1 | 0 | dioxane | 72 | 38 | 98 |
| 5 | 16a | 0.3 | 0 | dioxane | 72 | 50 | 99 |
| 6 | 16a | 0.3 | 10 | dioxane | 48 | 75 | 98 |
| 7 | 18a | 0.1 | 0 | THF | 72 | 38 | 99 |
| 8 | 18a | 0.2 | 10 | dioxane | 72 | 64 | 94 |
| 9 | 18a | 0.1 | 25 | THF | 48 | 63 | 90 |
| 10 | 18a | 0.3 | 10 | dioxane | 48 | 84 | 99 |
| 11 | 18a | 0.5 | 10 | dioxane | 48 | 79 | 98 |
The reactions were carried out using 5 equiv of borane stabilized with NaBH4. Isolated yield of amides by column chromatography.
Determined by GC of acetyl derivatives on a chiral column (CP-Chirasil-Dex-CB).
