Abstract
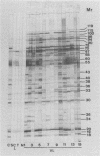
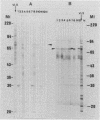
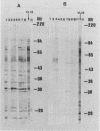
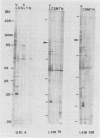
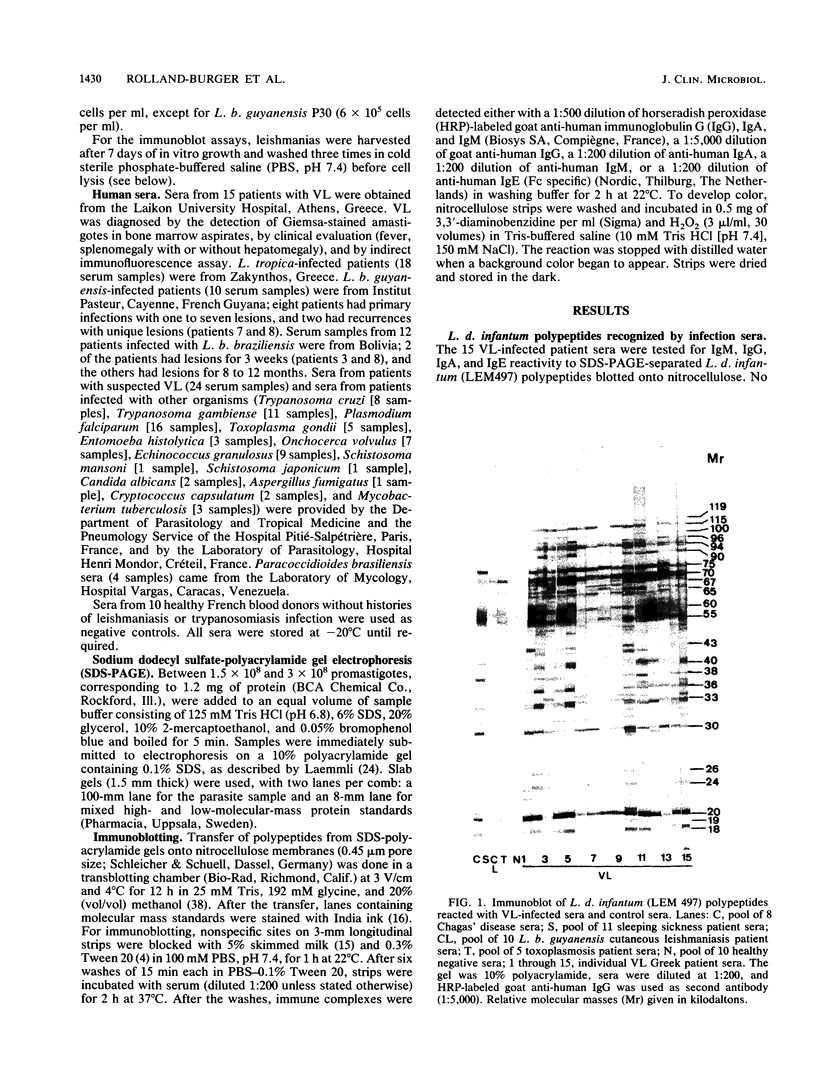
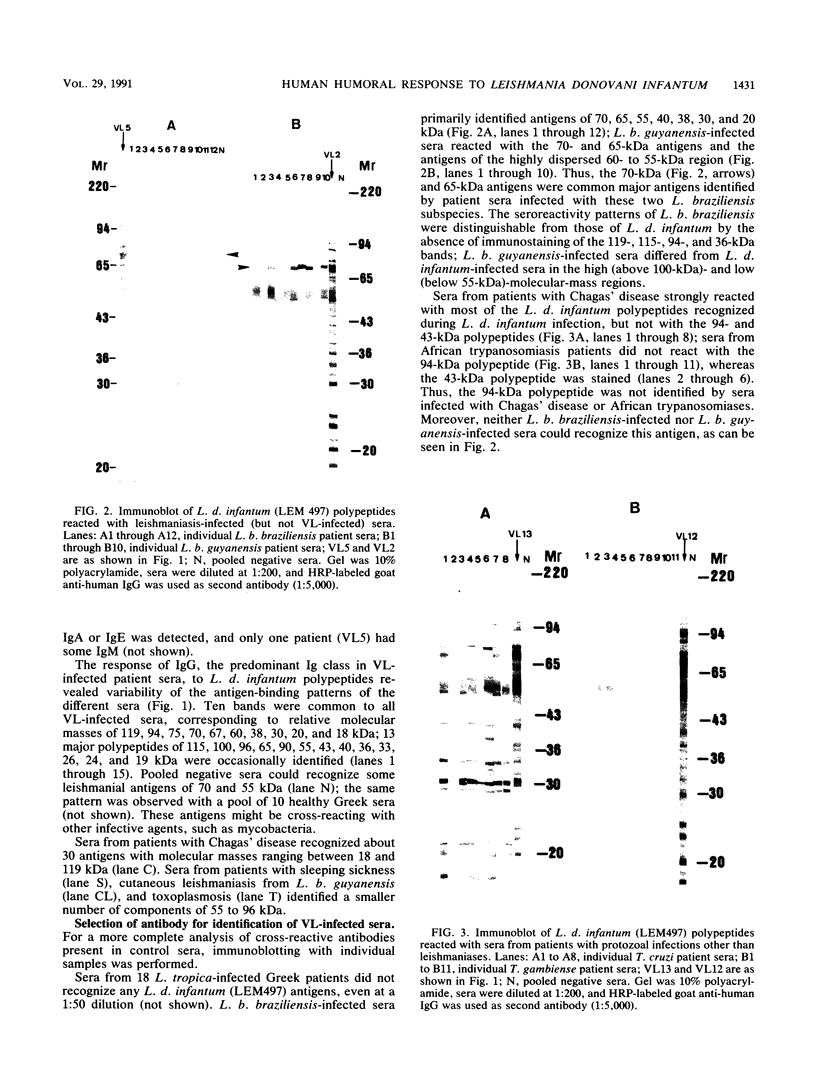
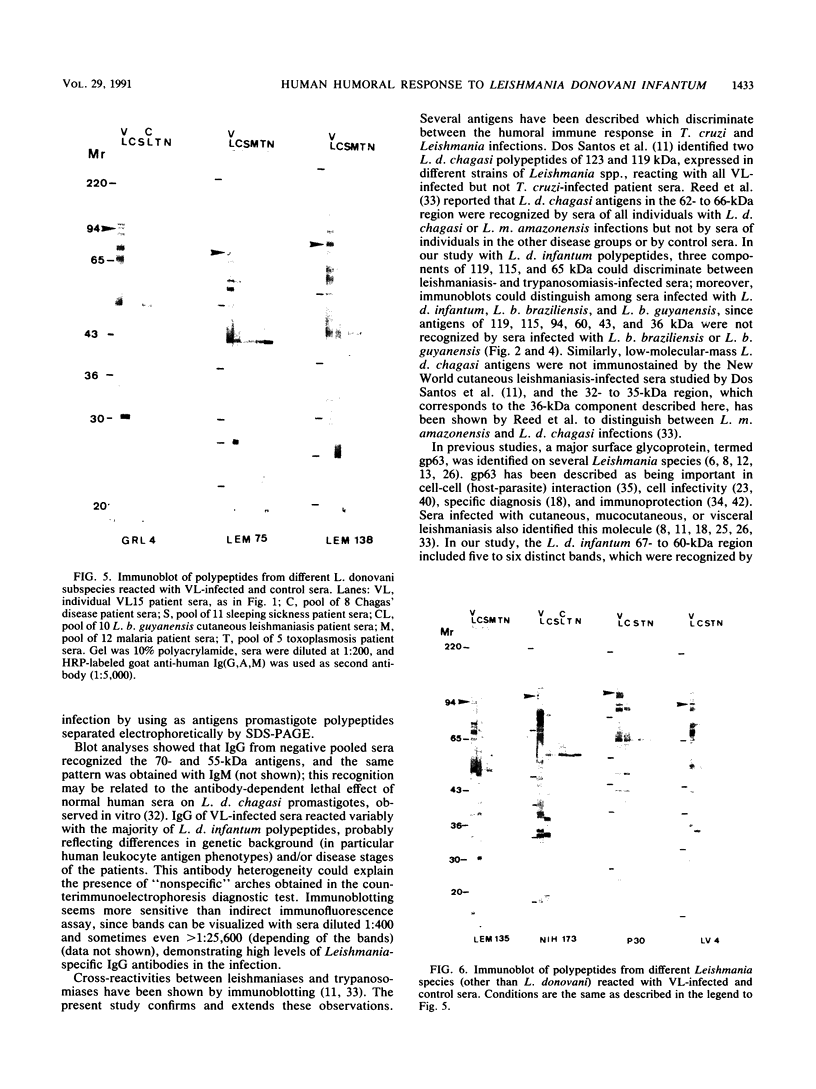
Using the immunoblot technique, we have compared the reactions of Leishmania donovani infantum polypeptides with the immunoglobulin G of human sera from patients with parasitologically proven L. d. infantum infection, with suspected visceral leishmaniasis, and with other leishmaniases, protozoiases, helminthiases, and fungal or bacterial diseases. A 94-kDa component reacted with all L. d. infantum-infected sera and with 75% of sera from patients with clinical and serological but no parasitological diagnoses. No reaction was observed with sera from patients in the other disease groups or with control sera. Studies of eight different isolates, subspecies, and species of the genus Leishmania demonstrated that the 94-kDa component was expressed in all strains examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afchain D., Le Ray D., Fruit J., Capron A. Antigenic make-up of Trypanosoma cruzi culture forms: identification of a specific component. J Parasitol. 1979 Aug;65(4):507–514. [PubMed] [Google Scholar]
- Anthony R. L., Williams K. M., Sacci J. B., Rubin D. C. Subcellular and taxonomic specificity of monoclonal antibodies to New World Leishmania. Am J Trop Med Hyg. 1985 Nov;34(6):1085–1094. doi: 10.4269/ajtmh.1985.34.1085. [DOI] [PubMed] [Google Scholar]
- Badaró R., Reed S. G., Barral A., Orge G., Jones T. C. Evaluation of the micro enzyme-linked immunosorbent assay (ELISA) for antibodies in American visceral leishmaniasis: antigen selection for detection of infection-specific responses. Am J Trop Med Hyg. 1986 Jan;35(1):72–78. doi: 10.4269/ajtmh.1986.35.72. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Blaxter M. L., Miles M. A., Kelly J. M. Specific serodiagnosis of visceral leishmaniasis using a Leishmania donovani antigen identified by expression cloning. Mol Biochem Parasitol. 1988 Sep;30(3):259–270. doi: 10.1016/0166-6851(88)90095-3. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Camargo M. E., Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969 Jul;18(4):500–505. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock K. M., Hodgen A. N., Channon J. Y., Siongok T. K., Lucas S. B., Rees P. H. Enzyme-linked immunosorbent assay (ELISA) for the diagnosis of visceral leishmaniasis in Kenya. J Infect Dis. 1985 Apr;151(4):750–752. doi: 10.1093/infdis/151.4.750. [DOI] [PubMed] [Google Scholar]
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Jaffe C. L., Dwyer D. M. Identification of cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect Immun. 1984 Feb;43(2):637–643. doi: 10.1128/iai.43.2.637-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães M. C., Celeste B. J., de Castilho E. A., Mineo J. R., Diniz J. M. Immunoenzymatic assy (ELISA) in mucocutaneous leishmaniasis, kala-azar, and Chagas' disease: an epimastigote Trypanosoma cruzi antigen able to distinguish between anti-Trypanosoma and anti-Leishmania antibodies. Am J Trop Med Hyg. 1981 Sep;30(5):942–947. doi: 10.4269/ajtmh.1981.30.942. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Bucher K. Immunoblotting with monoclonal antibodies: importance of the blocking solution. Anal Biochem. 1986 Dec;159(2):386–389. doi: 10.1016/0003-2697(86)90357-x. [DOI] [PubMed] [Google Scholar]
- Heath S., Chance M. L., Hommel M., Crampton J. M. Cloning of a gene encoding the immunodominant surface antigen of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Apr;23(3):211–222. doi: 10.1016/0166-6851(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Hedge E. C., Moody A. H., Ridley D. S. An easily cultured Crithidia sp. as antigen for immunofluorescence for kala-azar. Trans R Soc Trop Med Hyg. 1978;72(4):445–445. doi: 10.1016/0035-9203(78)90154-2. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81(4):587–594. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Use of purified parasite proteins from Leishmania donovani for the rapid serodiagnosis of visceral leishmaniasis. J Infect Dis. 1988 Jun;157(6):1212–1220. doi: 10.1093/infdis/157.6.1212. [DOI] [PubMed] [Google Scholar]
- Kweider M., Lemesre J. L., Santoro F., Kusnierz J. P., Sadigursky M., Capron A. Development of metacyclic Leishmania promastigotes is associated with the increasing expression of GP65, the major surface antigen. Parasite Immunol. 1989 May;11(3):197–209. doi: 10.1111/j.1365-3024.1989.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemesre J. L., Rizvi F. S., Afchain D., Sadigursky M., Capron A., Santoro F. Subspecies-specific surface antigens of promastigotes of the Leishmania donovani complex. Infect Immun. 1985 Oct;50(1):136–141. doi: 10.1128/iai.50.1.136-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J., Spithill T. W., Handman E. Characterization of integral membrane proteins of Leishmania major by Triton X-114 fractionation and analysis of vaccination effects in mice. Infect Immun. 1989 Jul;57(7):2203–2209. doi: 10.1128/iai.57.7.2203-2209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas M. G., Hajkowski R., Hockmeyer W. T. Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J Immunol Methods. 1983 Nov 11;64(1-2):205–214. doi: 10.1016/0022-1759(83)90399-x. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Badaro R., Lloyd R. M. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol. 1987 Mar 1;138(5):1596–1601. [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Sheppard H. W., Dwyer D. M. Cloning of Leishmania donovani genes encoding antigens recognized during human visceral leishmaniasis. Mol Biochem Parasitol. 1986 Apr;19(1):35–43. doi: 10.1016/0166-6851(86)90063-0. [DOI] [PubMed] [Google Scholar]
- Smrkovski L. L., Larson C. L. Antigenic cross-reactivity between Mycobacterium bovis (BCG) and Leishmania donovani. Infect Immun. 1977 Nov;18(2):561–562. doi: 10.1128/iai.18.2.561-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. C., Jr, McMahon-Pratt D. Purification and characterization of an 80-kilodalton membrane protein from Leishmania donovani. Infect Immun. 1988 Sep;56(9):2385–2391. doi: 10.1128/iai.56.9.2385-2391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K., Donelson J. E. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J Immunol. 1989 Jul 15;143(2):678–684. [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]
- dos Santos J. I., Morgado M. G., Galvão-Castro B. Human visceral leishmaniasis: analysis of the specificity of humoral immune response to polypeptides of Leishmania donovani chagasi. Am J Trop Med Hyg. 1987 Sep;37(2):263–270. doi: 10.4269/ajtmh.1987.37.263. [DOI] [PubMed] [Google Scholar]