Table 1.
Asymmetric Intramolecular Stetter Reaction of Mono-Substituted Cyclohexadienones
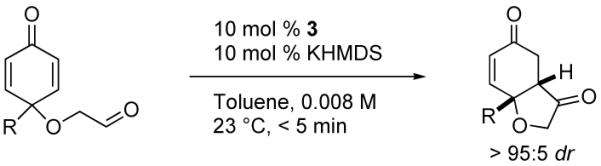 | |||||
|---|---|---|---|---|---|
| entrya | R | substrate | product | Yield (%) | ee (%)b |
| 1 | Me | 1 | 2 | 90 | 92 |
| 2 | Et | 6 | 7 | 86 | 94 |
| 3 | iPr | 8 | 9 | 87 | 94 |
| 4 | tBu | 10 | 11 | 86 | 94 |
| 5 | Ph | 12 | 13 | 87 | 88 |
| 6 | 4-BrPh | 14 | 15 | 78 | 85 |
| 7 | CH2OAc | 16 | 17 | 86 | 83 |
| 8 | CH2CH2OMe | 18 | 19 | 86 | 82 |
| 9 | CH2CH2CO2Me | 20 | 21 | 94 | 87 |
All reactions conducted in the presence of 10 mol% catalyst and 10 mol% KHMDS in toluene at 23 °C.
Determined by HPLC or GC using a chiral stationary phase.
