Fig. 5.
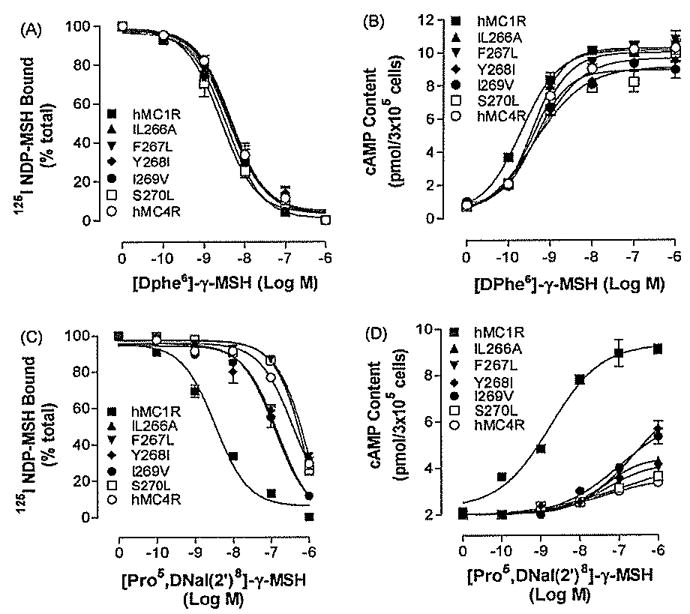
Binding affinities and potencies of [DPhe6]- γ-MSH and [Pro5, DNaI (2′)8]- γ-MSH at mutation of the non-conserved TM6 amino acid residues of hMC4R, Panel A depicts the binding affinity of [DPhe6]-γ-MSH as determined by inhibition of 125I NDP-MSH binding at the mutated receptors. Panel B represents the ability of [DPhe6]- γ-MSH to stimulate the production of intracellular cAMP at the mutated receptors. Pane C depicts the binding affinity of [Pro5, DNaI (2′)8]- γ-MSH as determined by inhibition of125I NDP-MSH binding at the mutated receptors. Panel D represents the ability of [Pro5, DNaI (2′)8]- γ-MSH to stimulate the production of intracellular cAMP at the mutated receptors. Data points represent the mean ± S.E.M. of at least three independent experiments.
