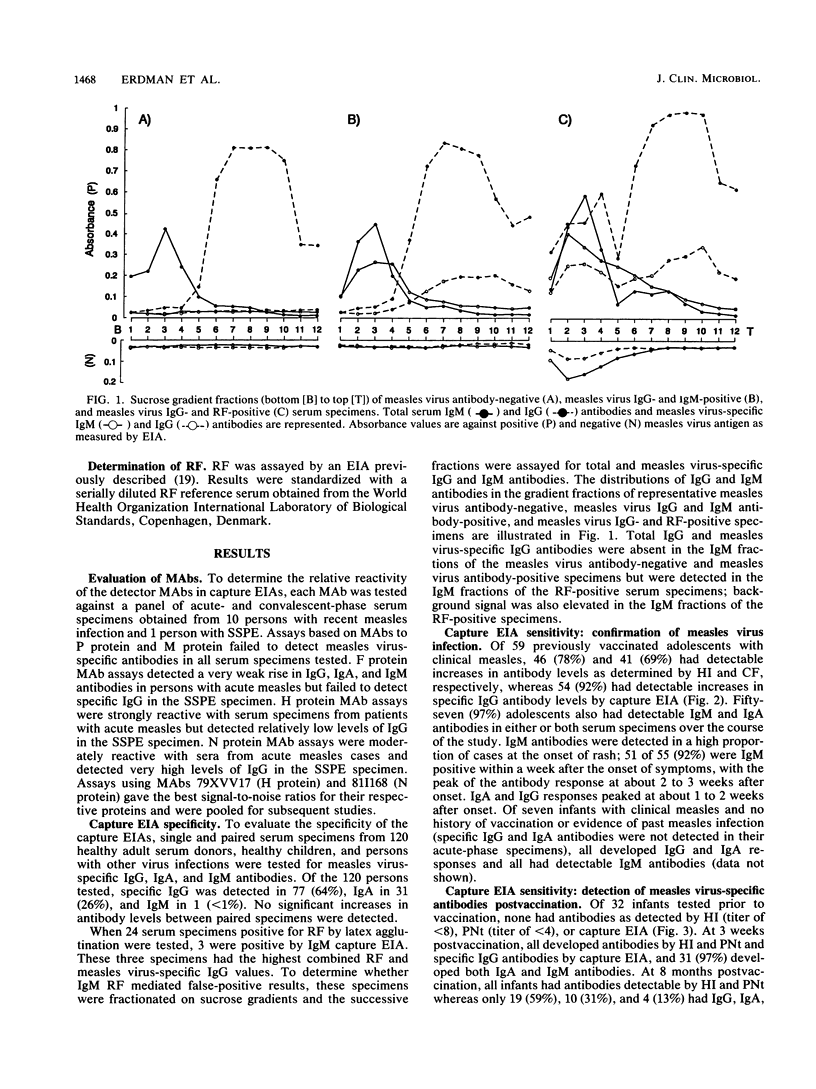
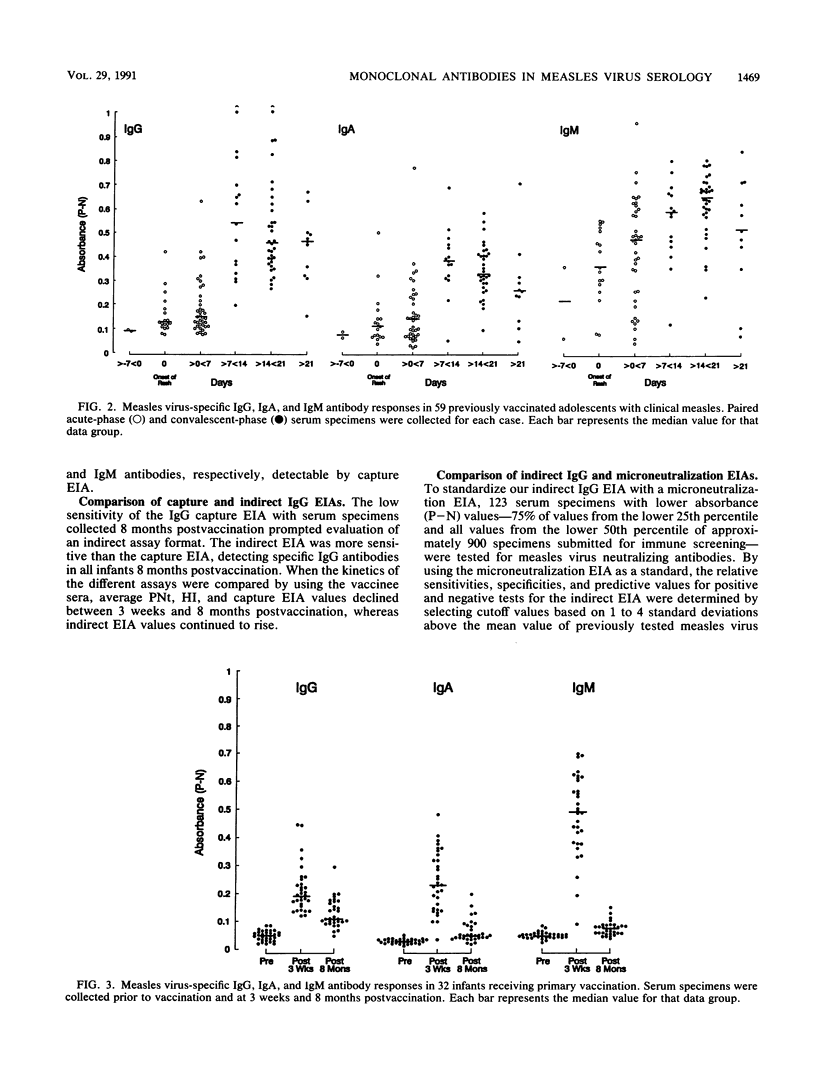
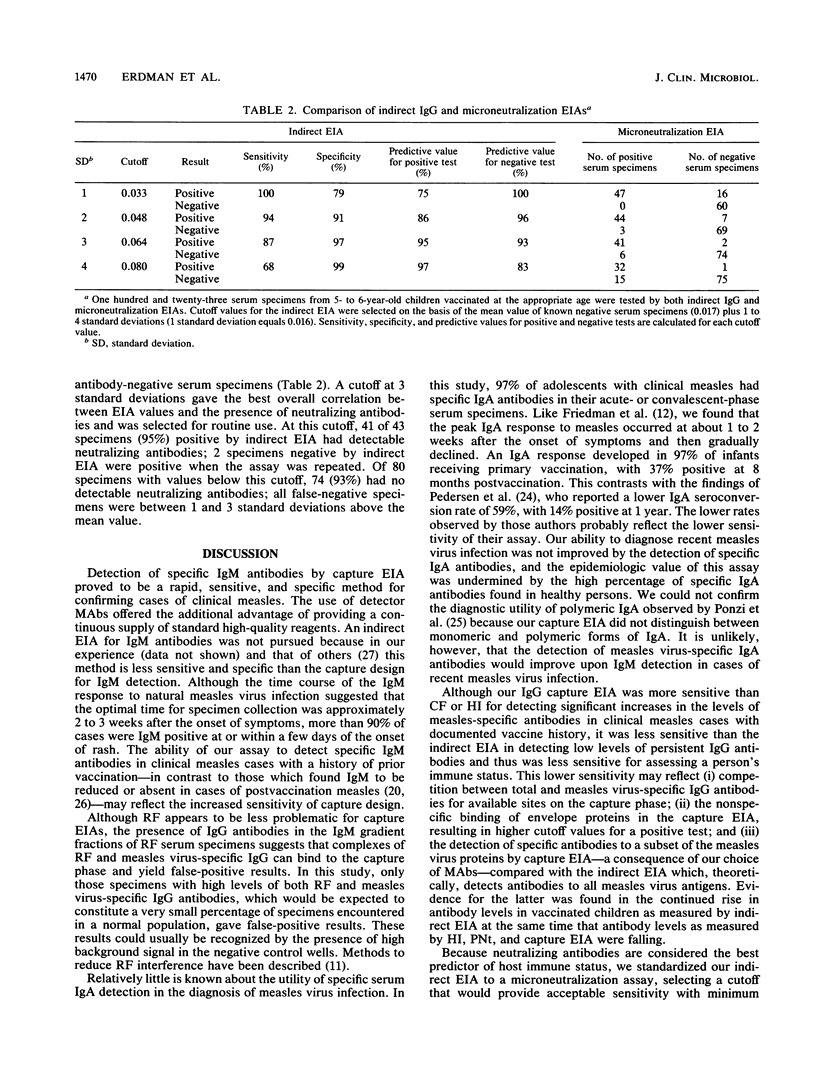
Abstract
Monoclonal antibodies to the hemagglutinin protein, fusion protein, phosphoprotein, matrix protein, and nucleoprotein of measles virus were evaluated as detector antibodies in capture enzyme immunoassays (EIAs) for the detection of specific serum immunoglobulin G (IgG), IgA, and IgM antibodies to measles virus. A pool of monoclonal antibodies to hemagglutinin protein and nucleoprotein proved optimal and was further evaluated. Specific IgM was detected in 97% of adolescents with clinical measles, 97% of infants 3 weeks postvaccination, and less than 1% of normal serum specimens. Specific IgA antibodies were found in 97% of adolescents with clinical measles, 97% of infants 3 weeks postvaccination, and less than 1% of normal serum specimens. Specific IgA antibodies were found in 97% of clinical measles cases and vaccinees, in 26% of healthy persons, and in 36% of infants 8 months postvaccination; consequently, IgA antibodies were not a useful indicator of recent measles infection. A significant increase in IgG antibodies between paired specimens was detected in 92% of clinical cases and all vaccinees. Only 59% of infant specimens had persistent IgG antibodies as detected by capture EIA at 8 months postvaccination, whereas all specimens had antibodies as detected by hemagglutination inhibition and plaque neutralization. An alternative indirect EIA, in which antigen was directly absorbed to the solid phase, was more sensitive than the capture design, detecting IgG antibodies in all infants postvaccination. When standardized with a microneutralization assay for the detection of persistent antibodies, the indirect IgG EIA gave predictive values for positive and negative tests exceeding 90%. Our capture IgM and indirect IgG EIAs provide a practical combination of serologic tests for the determination of acute measles virus infection and past exposure to measles virus or vaccine, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P., Herrmann K., Burns G. R. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981 Dec;3(5):251–260. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Bingham P. G., Stone Y. O. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol. 1985 Dec;22(6):1050–1052. doi: 10.1128/jcm.22.6.1050-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Stone Y. O., Tsou C., Fernie B. F. Identification of epitopes on respiratory syncytial virus proteins by competitive binding immunoassay. J Clin Microbiol. 1986 Mar;23(3):475–480. doi: 10.1128/jcm.23.3.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila P., Vuorimaa T., Kalimo K., Halonen P., Viljanen M., Granfors K., Toivanen P. A solid-phase radioimmunoassay for IgG and IgM antibodies against measles virus. J Gen Virol. 1977 Jan;34(1):167–176. doi: 10.1099/0022-1317-34-1-167. [DOI] [PubMed] [Google Scholar]
- Bellini W., Englund G., Rammohan K., McFarlin D. E. Evaluation of the structural proteins of the hamster neurotropic strain of measles virus with monoclonal antibodies. J Neuroimmunol. 1986 Apr;11(2):149–163. doi: 10.1016/0165-5728(86)90116-5. [DOI] [PubMed] [Google Scholar]
- Boteler W. L., Luipersbeck P. M., Fuccillo D. A., O'Beirne A. J. Enzyme-linked immunosorbent assay for detection of measles antibody. J Clin Microbiol. 1983 May;17(5):814–818. doi: 10.1128/jcm.17.5.814-818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. H., Haire M., Hadden D. S. Measles immunoglobulins in subacute sclerosing panencephalitis. Br Med J. 1971 Jan 2;1(5739):23–25. doi: 10.1136/bmj.1.5739.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer N. E., Cossen C. K., Shell G., Diggs J., Gallo D., Schmidt N. J. Enzyme immunoassay versus plaque neutralization and other methods for determination of immune status to measles and varicella-zoster viruses and versus complement fixation for serodiagnosis of infections with those viruses. J Clin Microbiol. 1985 Jun;21(6):869–874. doi: 10.1128/jcm.21.6.869-874.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Myoraku C. K., Schmidt N. J. Use of monoclonal antibodies to human immunoglobulin M in "capture" assays for measles and rubella immunoglobulin M. J Clin Microbiol. 1983 Sep;18(3):652–657. doi: 10.1128/jcm.18.3.652-657.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J. Antigen requirements, sensitivity, and specificity of enzyme immunoassays for measles and rubella viral antibodies. J Clin Microbiol. 1979 Jun;9(6):657–664. doi: 10.1128/jcm.9.6.657-664.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. G., Phillip M., Dagan R. Virus-specific IgA in serum, saliva, and tears of children with measles. Clin Exp Immunol. 1989 Jan;75(1):58–63. [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettering J. D., Schmidt N. J., Lennette E. H. Improved glycine-extracted complement-fixing antigen for human cytomegalovirus. J Clin Microbiol. 1977 Dec;6(6):647–649. doi: 10.1128/jcm.6.6.647-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman M. B., Blackburn C. K., Zimmerman S. E., French M. L. Comparison of enzyme-linked immunosorbent assay for acute measles with hemagglutination inhibition complement fixation, and fluorescent-antibody methods. J Clin Microbiol. 1981 Aug;14(2):147–152. doi: 10.1128/jcm.14.2.147-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman M. B., Blackburn C. K., Zimmerman S. E., French M. L., Wheat L. J. Rapid diagnosis of measles using enzyme-linked immunosorbent assay for measles immunoglobulin M. Diagn Microbiol Infect Dis. 1983 Sep;1(3):205–213. doi: 10.1016/0732-8893(83)90019-6. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Cremer N. E. Detection of IgG antibodies to measles virus using monoclonal antibodies for antigen-capture in enzyme immunoassay. J Virol Methods. 1984 May;8(3):255–263. doi: 10.1016/0166-0934(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Lievens A. W., Brunell P. A. Specific immunoglobulin M enzyme-linked immunosorbent assay for confirming the diagnosis of measles. J Clin Microbiol. 1986 Sep;24(3):391–394. doi: 10.1128/jcm.24.3.391-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann C. C., Hegg M. E., Rotte T. C., Phair J. P., Schiff G. M. Measles IgM response during reinfection of previously vaccinated children. J Pediatr. 1973 May;82(5):798–801. doi: 10.1016/s0022-3476(73)80069-1. [DOI] [PubMed] [Google Scholar]
- Negro Ponzi A., Merlino C., Angeretti A., Penna R. Virus-specific polymeric immunoglobulin A antibodies in serum from patients with rubella, measles, varicella, and herpes zoster virus infections. J Clin Microbiol. 1985 Oct;22(4):505–509. doi: 10.1128/jcm.22.4.505-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Identification of measles virus-specific hemolysis-inihibiting antibodies separate from hemagglutination-inhibiting antibodies. Infect Immun. 1975 Feb;11(2):231–239. doi: 10.1128/iai.11.2.231-239.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein W. A., Albrecht P., Herrmann K. L., Bernier R., Bart K. J., Rovira E. Z. The plaque-neutralization test as a measure of prior exposure to measles virus. J Infect Dis. 1987 Jan;155(1):146–149. doi: 10.1093/infdis/155.1.146. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R., Antonsdottir A., Evald T., Mordhorst C. H. Detection of measles IgM antibodies by enzyme-linked immunosorbent assay (ELISA). Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):153–160. doi: 10.1111/j.1699-0463.1982.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R., Mordhorst C. H., Ewald T., von Magnus H. Long-term antibody response after measles vaccination in an isolated arctic society in Greenland. Vaccine. 1986 Sep;4(3):173–178. doi: 10.1016/0264-410x(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Smith F. R., Curran A. S., Raciti K. A., Black F. L. Reported measles in persons immunologically primed by prior vaccination. J Pediatr. 1982 Sep;101(3):391–393. doi: 10.1016/s0022-3476(82)80064-4. [DOI] [PubMed] [Google Scholar]
- Tuokko H. Comparison of nonspecific reactivity in indirect and reverse immunoassays for measles and mumps immunoglobulin M antibodies. J Clin Microbiol. 1984 Nov;20(5):972–976. doi: 10.1128/jcm.20.5.972-976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuokko H., Salmi A. Detection of IgM antibodies to measles virus by enzyme-immunoassay. Med Microbiol Immunol. 1983;171(4):187–198. doi: 10.1007/BF02123492. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E. Enzyme-immunoassays for antibodies in measles, cytomegalovirus infections and after rubella vaccination. Br J Exp Pathol. 1976 Apr;57(2):243–247. [PMC free article] [PubMed] [Google Scholar]
- Weigle K. A., Murphy M. D., Brunell P. A. Enzyme-linked immunosorbent assay for evaluation of immunity to measles virus. J Clin Microbiol. 1984 Mar;19(3):376–379. doi: 10.1128/jcm.19.3.376-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


