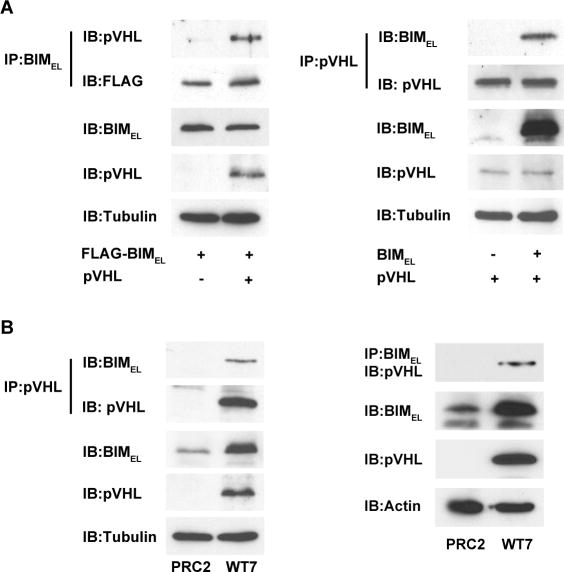
Figure 5. Co-immunoprecipitation of pVHL and BIMEL.
(A) COS-7 cells were transiently transfected with 1 μg plasmid encoding FLAG-tagged BIMEL with or without 1 μg pVHL plasmid (left), or with 1 μg pVHL plasmid with or without 1 μg BIMEL plasmid (right). The next day, whole cell lysates were prepared and 500 μg of each were subjected to immunoprecipitation with anti-BIM polyclonal antibodies or anti-pVHL monoclonal antibody as indicated. The resulting immune complexes were split in half and analyzed by immunoblotting with anti-pVHL and anti-FLAG monoclonal antibodies (left) or anti-BIM and anti-pVHL polyclonal antibodies (right). A portion of each lysate (25 μg) taken prior to immunoprecipitation was blotted to determine total levels of BIMEL, pVHL and α-tubulin (to control for loading). The pVHL and BIMEL in the immunoprecipitates and in the whole cell lysates were analyzed on the same blots and for the same exposure times. (B) PRC2 and WT7 cell lysates (500 μg) were subjected to immunoprecipitation with anti-pVHL monoclonal antibody or anti-BIM polyclonal antibodies as indicated and the immunoprecipitated proteins were then immunoblotted with anti-BIM and anti-pVHL polyclonal antibodies (left) or with anti-pVHL monoclonal antibody (right). A portion (25ug) of each lysate was taken prior to immunoprecipitation to assess total levels of BIMEL, pVHL and either α-tubulin or actin. The pVHL and BIMEL in the immunoprecipitates and in the whole cell lysates were analyzed on the same blots and for the same exposure times. For both A and B, similar results were obtained in 3-4 independent experiments.