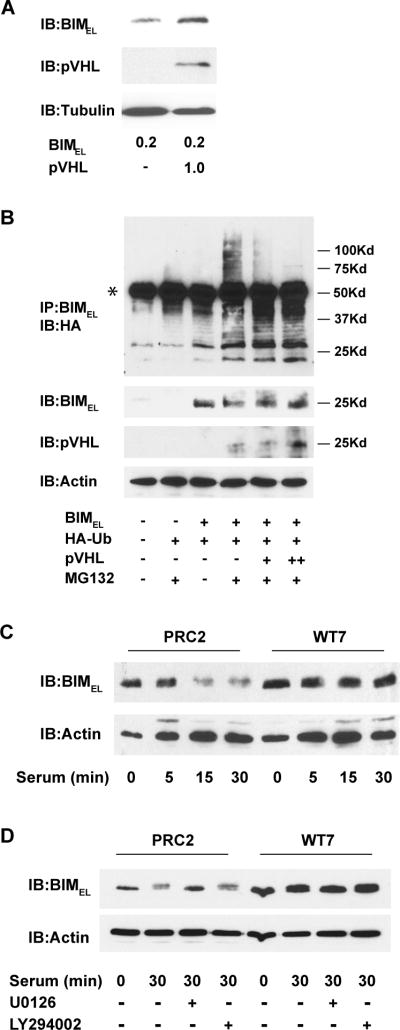
Figure 6. Effects of pVHL on BIMEL polyubiquitination and its stability after serum-stimulation.
(A) COS-7 cells were transiently transfected with 0.2 μg of BIMEL plasmid with or without 1 μg of pVHL plasmid. The next day, whole cell lysates were prepared and immunoblotted for BIMEL, pVHL and α-tubulin. Note that co-expressing pVHL results in an increase in the levels of BIMEL under these conditions. (B) COS-7 cells were transfected with HA-ubiquitin (1 μg), BIMEL (1 μg) and either 1 or 3 μg (+ or ++, respectively) of pVHL expression plasmid. After 24 h, the cells were lysed and immunoprecipitated with anti-BIM polyclonal antibodies. Polyubiquitinated BIMEL was detected by immunoblotting the immune complexes with anti-HA monoclonal antibody (the asterisk identifies the IgG heavy chain from the anti-Bim antibody used for the immunoprecipitation). Where indicated, the proteasome inhibitor MG132 (50 μM) was added to the cells 6 h prior to lysis. A portion of each cell lysate taken prior to immunoprecipitation was analyzed by immunoblotting for BIMEL, pVHL and actin. This experiment has been repeated twice and in each case the presence of pVHL resulted in a decrease in the amount of high molecular weight polyubiquitinated BIMEL. (C) PRC2 and WT7 cells were cultured in serum-free media overnight and then stimulated with fresh serum-containing media for 0, 5, 15, or 30 minutes. Whole cell lysates were prepared and subjected to immunoblotting with antibodies against BIM and actin. (D) Serum-starved PRC2 and WT7 cells were stimulated with fresh serum-containing media or left in serum-free media for 30 min in the presence or absence of the MEK inhibitor U0126 (50 μM) or phosphatidylinositol 3-kinase inhibitor LY294002 (50 μM). At the end of the treatment period, whole cell lysates were prepared and subjected to immunoblotting with antibodies against BIM and actin. Note the MEK-dependent decrease in BIMEL levels after serum stimulation in PRC2 cells and the absence of any decrease in similarly treated WT7 cells. Results shown in C and D are representative of those obtained in 3 independent experiments.