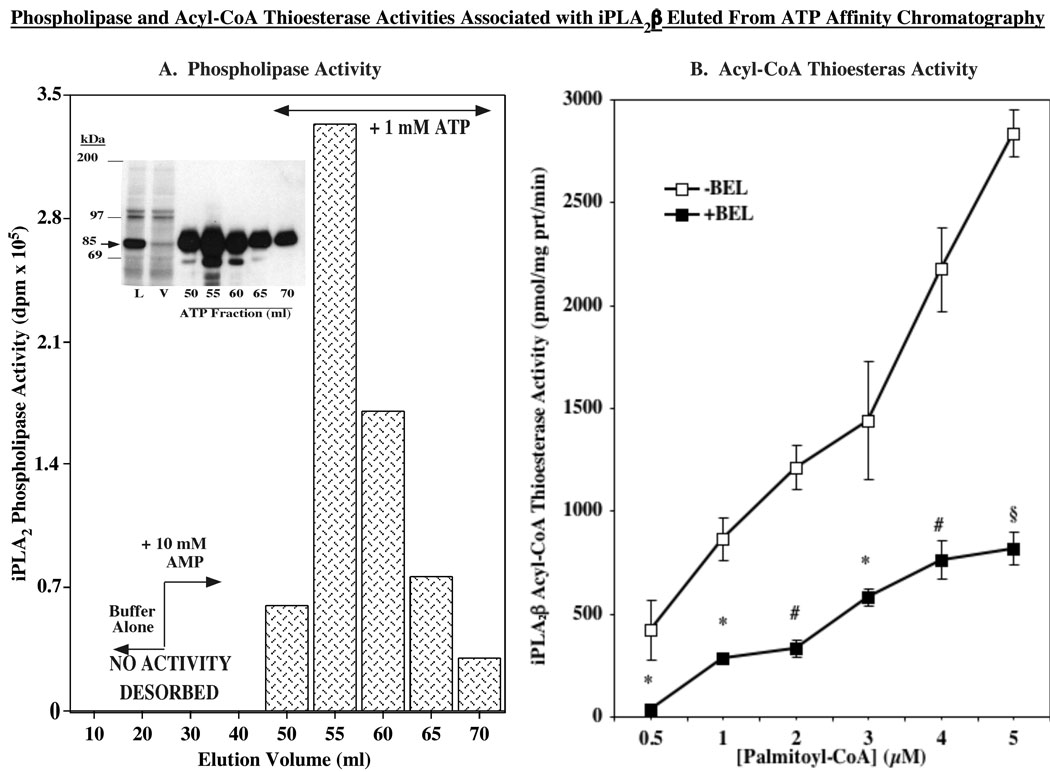
FIGURE 6. iPLA2β-associated phospholipase and acyl-CoA thioesterase activity.
A. Phospholipase activity. Partially-purified iPLA2β was prepared from iPLA2β-overexpressing INS-1 cells (30 × T225 flasks), as described (25). Ca2+-independent phospholipase activity of iPLA2β was determined in eluants from the ATP affinity column using [14C]-PLPC as the substrate. (Inset, immunoblotting analyses of iPLA2β in ATP column eluants). B. Acyl-CoA thioesterase activity. Fractions containing phospholipase activity were pooled, protein concentration determined, and acyl-CoA thioesterase specific activity was measured in 1 µg protein aliquots using [14C]-palmitoyl-CoA as substrate in the absence or presence of BEL (10 µM). (*,#,§BEL-treated group significantly different from control group, p < 0.05, p < 0.01, and p < 0.005, respectively, n=3).