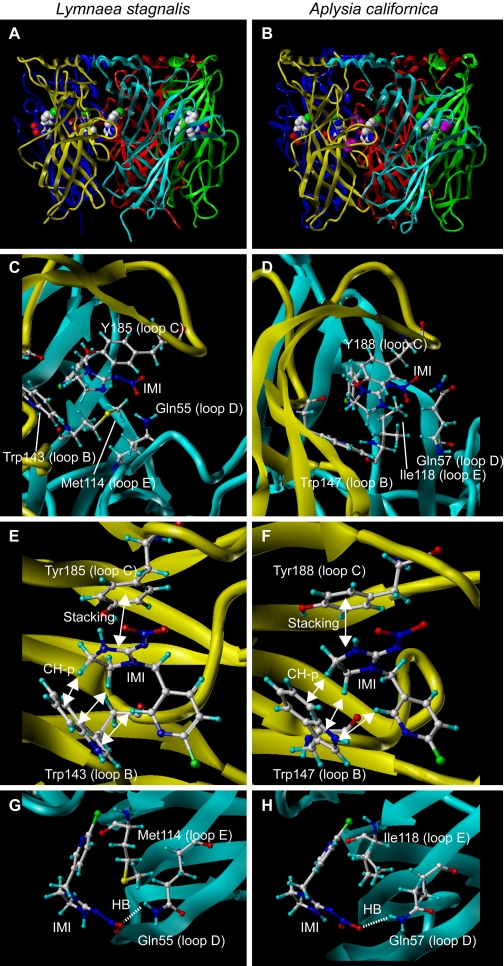
Fig. 5.
Amino acids interacting with a neonicotinoid imidacloprid in Ls- and Ac-AChBPs. A and B, side views of crystal structures of Ls- (A) and Ac- (B) AChBPs. All were prepared using Sybyl (version 7.1; Tripos Associates, Inc.). In A and B, imidacloprid and isopropyl alcohol (colored magenta) are generated in spheres to highlight. In C and D, amino acids interacting with imidacloprid in Ls- and Ac-AChBPs are shown, respectively. Irrespective of the mollusc species, a common mechanism is involved in the neonicotinoid recognition by the ligand binding domain of AChBPs. The main chain donating loops A to C is colored yellow, whereas the main chain giving loops D to F is shown cyan. In E (Ls-AChBP) and F (Ac-AChBP), amino acids from loops B and C are shown, whereas in G and H, those from loops D and E are shown in orientations facilitating view of interactions. Carbon, hydrogen, nitrogen, oxygen, and chlorine atoms are colored white, light blue, blue, red, and blue-green, respectively. IMI, imidacloprid; HB, hydrogen bond.