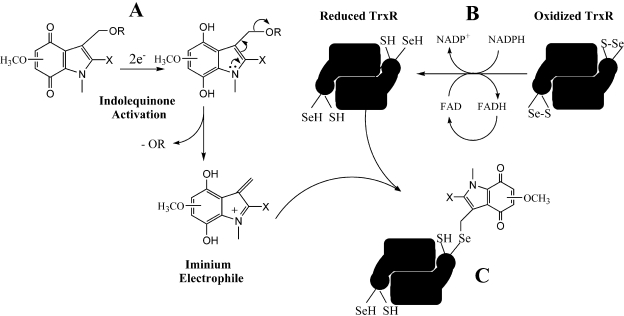
Fig. 7.
A proposed mechanism for the inhibition of TrxR by indolequinones. A, indolequinone activation by two-electron reduction to generate a reactive iminium electrophile. B, reduction of oxidized TrxR by NADPH to generate the reduced C-terminal selenocysteine. C, inhibition of TrxR via alkylation of the reduced C-terminal selenocysteine by the reactive indolequinone iminium electrophile.