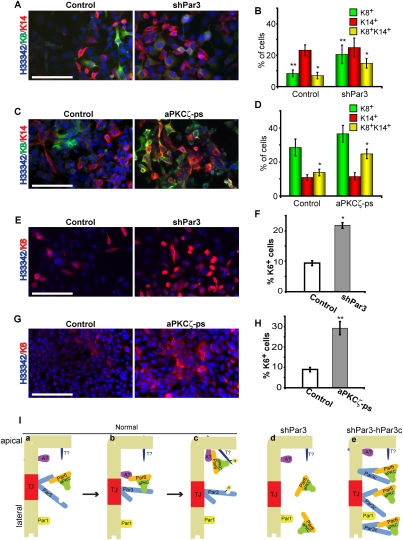
Figure 7.
Par3 and aPKC regulate progenitor differentiation. (A) Control or Par3-depleted COMMA-1D cells were grown for 7 d and stained for K8 and K14. (B) Quantification of A. (*) P = 0.005 (control vs. shPar3); (**) P = 0.01 (control vs. shPar3). (C) COMMA-1D cells were treated with 40 μM myristoylated aPKCζ pseudosubstrate every 48 h for 7 d, then stained for K8 and K14. (D) Quantification of C. (*) P = 0.003 (control vs. shPar3). (E) Control or Par3-depleted COMMA-1D cells were grown for 7 d and stained for K6. (F) Quantification of E. (*) P = 1.2 × 10−13 (control vs. shPar3). (G) COMMA-1D cells were treated with 40 μM myristoylated aPKCζ pseudosubstrate every 48 h for 7 d, then stained for K6. (H) Quantification of G. (**) P = 5.8 × 10−7 (control vs. aPKCζ-ps). Bars, 100 μm;error bars, SD; n = 4. (I) Model for Par3/aPKC function in MECs. Par3 permits the recruitment of the constitutive aPKC/Par6 complex to tight junctions (panel a), where the complex is handed off to an apical protein (panel b; apical protein [A?]) that is required for phosphorylating an apical target (panel c; apical target [T?]). (Panel d) In the absence of Par3, aPKC is not recruited to the plasma membrane and cannot phosphorylate apical targets. (Panel e) The Par3c variant can recruit aPKC to the plasma membrane, but cannot hand off aPKC appropriately, so both proteins remain bound and diffuse onto the apical and lateral surfaces, but aPKC cannot phosphorylate its normal targets. See the Discussion for additional details.