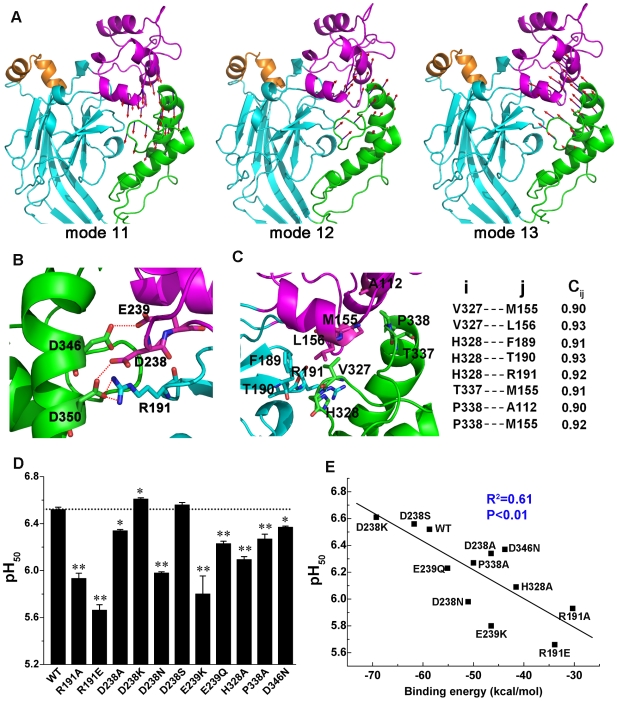
Figure 5. Correlation between the collective motions of thumb with finger and the channel gating.
(A) Cooperative motions of the thumb (green) with finger (magenta). These motions exist in most of the NMA modes. The motions in modes 11, 12, and 13 are shown as examples of such collective motions. The vector arrow represents the amplitude and direction of the displacement experienced by each residue on the interface between the two subdomains. The arrows mapped onto the structure clearly display the collective motions between the thumb and finger. (B) Hydrogen bonds between the thumb and finger domains. Key residues include and are represented in stick view. The dashed red lines represent hydrogen bonds. (C) Close-up view of the hydrophobic interface between the thumb and finger (left). The cross-correlation coefficients (C ij) of some important residue pairs between thumb (i) and finger (j) (right). (D) pH50 values for the WT channel and mutants (R191A, R191E, D238A, D238K, D238N, D238S, E239K, E239Q, H328A, P338A, D346N) that were designed to test key hydrogen bonding and hydrophobic interactions. Data are from five and three separate patches for kinetic and dose–response experiments, respectively. Student's t-test analysis shows that the differences in pH50 for the WT and mutant channels are statistically significant. *, p<0.05 versus WT; **, p<0.001 versus WT. (E) A linear correlation exists between binding free energies (ΔGbinding) of thumb and finger domain interactions and their corresponding pH50 values for the WT and the mutant channels described in (D). The binding free energies between the thumb and finger of the WT and all the mutants were calculated using the MM-PBSA method encoded in AMBER, version 9.0 [39]. The detailed computational procedure is described in the Text S1.