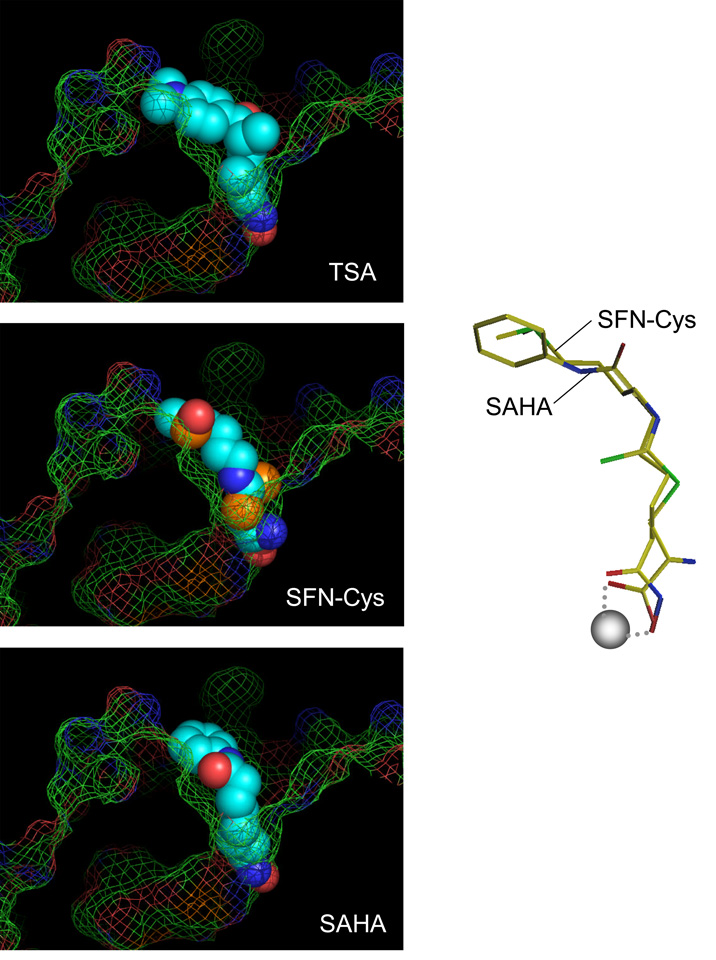
Fig. 1.
Inhibitors in the HDAC pocket. Binding of TSA (top) and SAHA (bottom) were from structural studies [Finnin et al., 1999]. Accelrys Insight II software was used to model interactions of putative inhibitors, with the following parameters: bidentate binding of the ligand to the zinc atom; H-bond partners for buried polar atoms; avoiding steric conflicts between ligand and enzyme based on a fixed protein; maintaining favorable torsion angles; following the favored positions of TSA and SAHA. SFN-Cys fit the HDAC pocket (center) and had comparable geometry to SAHA in the active site (right), with the α-carboxyl group of the cysteine moiety forming a bidentate ligand with the buried zinc atom (gray sphere). For full details, see [Myzak et al., 2004].