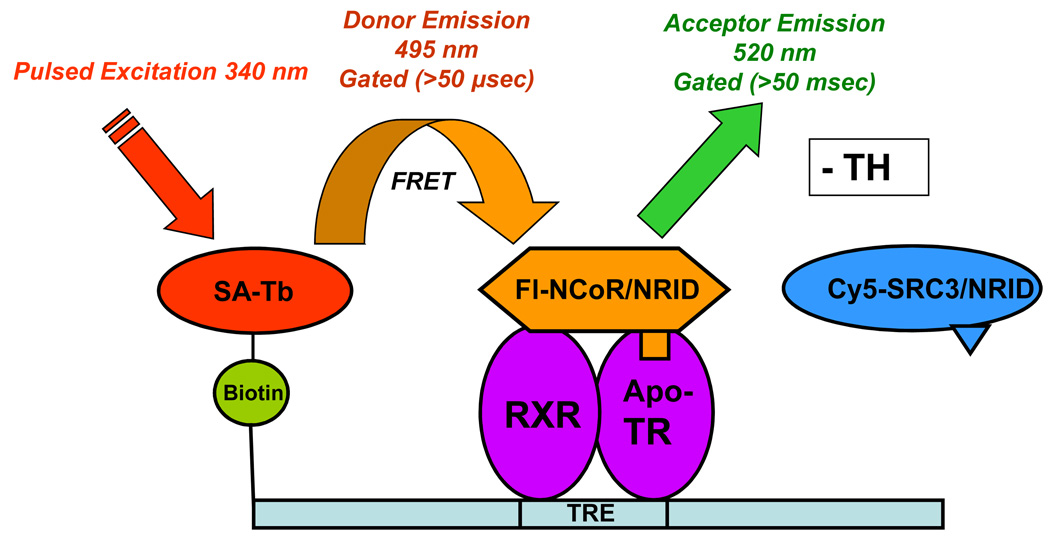
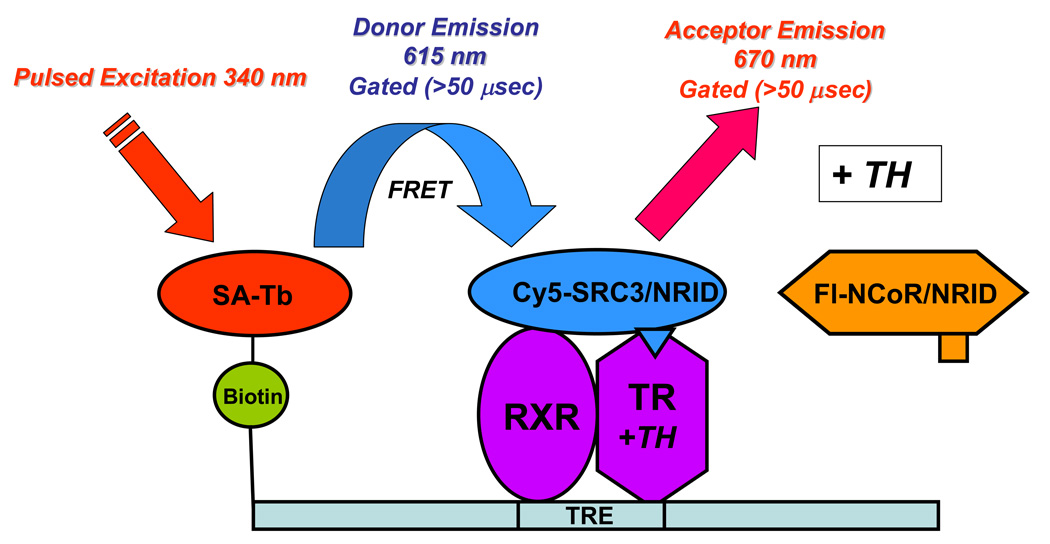
Figure 2.
Principle of the dual tr-FRET assays. A heterodimer of hTRβ (residues 82–456) and hRXRα (full length) was assembled onto a terbium-labeled streptavidin-bound biotinylated DR+4 TRE (49 bp from the rat myosin heavy chain promoter). (A) Unliganded (− TH) TR•RXR bound to a TRE interacts with NCoR fluorescein-labeled NRID fragment of mNCoR, residues 2057–2453. The Tb donor is excited at 340 nm, and the tr-FRET is assessed after 50-µs delay at 495 nm for terbium (donor) and at 520 nm for fluorescein (acceptor). (B) Liganded (+ TH) TR•RXR bound to a TRE interacts with Cy5-labeled hSRC3, residues 627–829. The Tb donor is excited at 340 nm, and the tr-FRET is assessed after 50-µs delay at 495 nm for terbium (donor) and at 670 nm for Cy5 (acceptor).