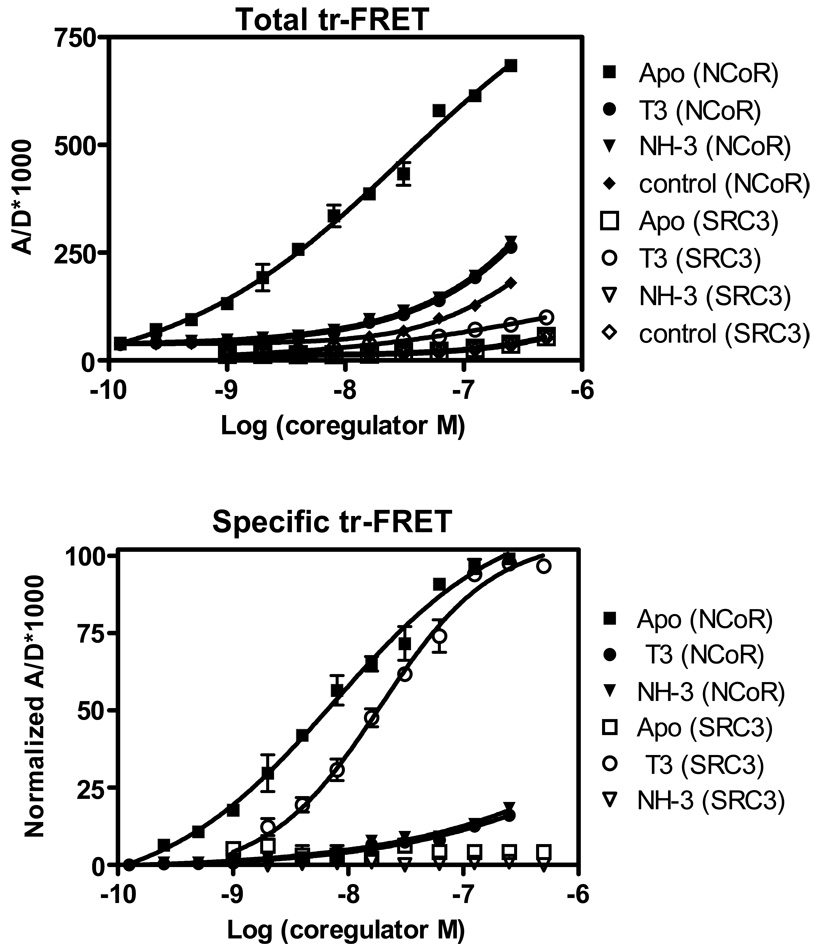
Figure 3.
Simultaneous coregulator titration assay – measurement of coregulator binding affinity to TR complexes. Measurement NCoR and SRC3 binding affinities for unliganded and liganded TRE-bound TR•RXR. (A) A fixed amount of TR•RXR (15 nM) bound to a biotinylated DR+4 TRE was incubated with increasing concentrations of both fluorescein-labeled NCoR and Cy5-labeled SRC3 in the absence of (Apo) and in the presence of 3 µM T3 and 25 µM NH-3, and in the absence of biotinylated TRE (control). Measured tr-FRET values are plotted as the ratio of acceptor to donor × 1000 vs. the log of coregulator concentrations. Note, the binding of SRC3 with TR unliganded [Apo (SRC3)], or treated with NH-3 [NH-3 (SRC3)] or the control are similar and appear superimposed. (B) The tr-FRET values in panel A were corrected for the corresponding diffusion-enhanced FRET values (control). The control corrected Bmax values of SRC3 (T3-TR) and NCoR (Apo TR) were set 100%, and the resulting normalized tr-FRET values are shown. Three independent sets of experiments were performed in replicate, and each assay point in the binding curves represents the mean ± standard deviation of six measurements. Results in panel B were analyzed by nonlinear regression with an equation for the sigmoidal dose response (variable slope) in GraphPad Prism, and the concentrations of fluorophore-labeled NCoR and SRC3 at 50% (EC50) of maximal binding in the presence of T3 and NH-3 were obtained and expressed as mean ± standard deviation of three different experiments.