Annals of Botany100: 1491–1505, 2007
There were problems in the reproduction of some figures in this article. The entire article is reproduced here with the original page numbers. The publishers would like to apologize for these problems.
Annals of Botany100: 1491–1505, 2007
There were problems in the reproduction of some figures in this article. The entire article is reproduced here with the original page numbers. The publishers would like to apologize for these problems.
Molecular phylogenies have suggested a new circumscription for Fabales to include Leguminosae, Quillajaceae, Surianaceae and Polygalaceae. However, recent attempts to reconstruct the interfamilial relationships of the order have resulted in several alternative hypotheses, including a sister relationship between Quillajaceae and Surianaceae, the two species-poor families of Fabales. Here, floral morphology and ontogeny of these two families are investigated to explore evidence of a potential relationship between them. Floral traits are discussed with respect to early radiation in the order.
Floral buds of representatives of Quillajaceae and Surianaceae were dissected and observed using light microscopy and scanning electron microscopy.
Quillajaceae and Surianaceae possess some common traits, such as inflorescence morphology and perianth initiation, but development and organization of their reproductive whorls differ. In Quillaja, initiation of the diplostemonous androecium is unidirectional, overlapping with the petal primordia. In contrast, Suriana is obdiplostemonous, and floral organ initiation is simultaneous. Independent initiation of five carpels is common to both Quillaja and Suriana, but subsequent development differs; the antesepalous carpels of Quillaja become fused proximally and exhibit two rows of ovules, and in Suriana the gynoecium is apocarpous, gynobasic, with antepetalous biovulate carpels.
Differences in the reproductive development and organization of Quillajaceae and Surianaceae cast doubt on their potential sister relationship. Instead, Quillaja resembles Leguminosae in some floral traits, a hypothesis not suggested by molecular-based phylogenies. Despite implicit associations of zygomorphy with species-rich clades and actinomorphy with species-poor families in Fabales, this correlation sometimes fails due to high variation in floral symmetry. Studies considering specific derived clades and reproductive biology could address more precise hypotheses of key innovation and differential diversification in the order.
Key words: Fabales, Leguminosae, eurosids I, floral ontogeny, Polygalaceae, Quillajaceae, Surianaceae, floral symmetry
The order Fabales is part of one of the major clusters of rosids known as fabids or eurosids I; it forms part of the nitrogen-fixing clade, together with Cucurbitales, Fagales and Rosales (Soltis et al., 1995; Savolainen et al., 2000a, b; APG, 2003). Molecular-based angiosperm phylogenies (e.g. Chase et al., 1993; Soltis et al., 1997) circumscribe Fabales to include two species-rich families, Leguminosae (20 000 species in 727 genera; Lewis et al., 2005) and Polygalaceae (1000/21; Eriksen and Persson, 2007), and two species-poor families, Quillajaceae (2/1; Kubitzki, 2007) and Surianaceae (8/5; Schneider, 2007). Prior to molecular studies (Fernando et al., 1993; Morgan et al., 1994), Quillaja and Surianaceae were usually placed within, or related to, Rosaceae and Simaroubaceae, respectively (Dahlgren, 1980; Cronquist, 1981; Thorne, 1992; Takhtajan, 1997), rather than with Polygalaceae or Leguminosae. However, independent anatomical, biochemical, caryological, embryological and morphological studies on Quillaja and Surianaceae have highlighted important differences from Rosaceae and Simaroubaceae (Edman, 1936; Bate-Smith, 1965; Prance, 1965; Nooteboom, 1966; Mitchell and Geismann, 1971; Eyde, 1975; Goldblatt, 1976; Fehrenbach and Barthlott, 1988; Fernando and Quinn, 1992; Zhang, 1992; Heo and Tobe, 1994; Behnke et al., 1996).
Relationships within Fabales are currently poorly defined. Recent molecular analyses of Fabales using relatively high sampling and both single and combined data sets of matK and rbcL sequences have suggested five contrasting hypotheses for interfamilial relationships (Fig. 1A–E), all with low or moderate support (M. A. Bello et al., unpubl. res.). Quillajaceae and Surianaceae are sister families in two of these hypotheses. Despite the lack of resolution within Fabales, Leguminosae, Polygalaceae and Surianaceae appear as monophyletic entities with high support (Quillajaceae was represented by Quillaja saponaria alone). This fact, together with phylogenetic age estimates for the families, suggests that the main lineages of the order could have undergone rapid radiation before the establishment of the families (M. A. Bello et al., unpubl. res.).
Alternative interfamilial hypotheses derived from molecular analyses based on plastid data. (A–C) Parsimony-based strict consensus trees from (A) rbcL, (B) matK and (C) combined matK + rbcL data sets. (D, E) Majority-rule consensus trees derived from Bayesian analyses of (D) rbcL and (E) matK and combined matK + rbcL data sets.
For rosids in general, more data are required to understand the evolution of morphological features in the novel large clades that have emerged from molecular studies (Matthews and Endress, 2002, 2006; Soltis and Soltis, 2004; Endress and Friis, 2006; Endress and Matthews, 2006; Schönenberger and von Balthazar, 2006). Phenotypic characters are the features that ultimately support the distinctiveness and historical relationships of real biological entities, and represent alternative evidence that merits exploration, particularly in cases of suspected clade radiation (Donoghue and Sanderson, 1992; Bateman, 1999; Bateman and DiMichele, 2002).
The floral morphology and ontogeny of the small families Quillajaceae and Surianaceae are examined here in order to evaluate hypotheses of relationships and to discuss the differential diversification of the families of Fabales in relation to floral symmetry. This investigation represents part of a wider survey of floral structure and ontogeny in Fabales (M. A. Bello et al., unpubl. res.). Within the order, earlier published studies of floral morphology and ontogeny are mainly restricted to Polygalaceae (van der Meijden, 1982; Kruger et al., 1988; Bamert, 1990; Eriksen, 1993; Prenner, 2004b) and a wide range of Leguminosae (Tucker, 1991, 1998, 2000a, 2003; Tucker and Kantz, 1997; Klitgaard, 1999; Herendeen et al., 2003; Prenner, 2004a). These studies offer a valuable comparative framework for the present data.
Quillajaceae includes a single genus, Quillaja, with two species, one from Brazil (Quillaja brasiliensis) and the other from the Andes of Peru and Chile (Quillaja saponaria). They are trees with alternate and simple leaves, caducous stipules, and fruits forming multifollicles with winged seeds (Sterling, 1966; Fuks, 1983; Morgan et al., 1994; Takhtajan, 1997; Kubitzki, 2007). Quillaja is widely exploited economically by the pharmaceutical and the cosmetic industries, due the presence of saponins in the inner bark (Takhtajan, 1997; Guo et al., 1998; Suzuki et al., 2002; Kim et al., 2006). Surianaceae is a woody family of eight species in five genera, Cadellia, Guilfoylia and Stylobasium from Australia, Recchia from Mexico and the pantropical Suriana (Cronquist, 1981; Fernando et al., 1993; Crayn et al., 1995; Takhtajan, 1997). Surianaceae possess alternate, non-stipulate simple leaves (except Recchia with compound leaves), the fruits are drupaceous, baccate or bony nuts, with curved/folded embryos and scarce or absent endosperm (Loesener and Solereder, 1905; Gutzwiller, 1961; Cronquist, 1983; Juàrez-Sierra, 1988; Daniel et al., 1997; Takhtajan, 1997).
Floral buds of two species were collected at several different stages of development: Quillaja saponaria Molina (living collections Royal Botanic Gardens, Kew 1973-4810, Royal Botanic Gardens, Edinburgh 1998-1562, and the wild-source collection Zapata s.n. from Chile), and Suriana maritima L. (Ronse Decraene 1422). Buds were fixed in Formalin, Acetic acid and Alcohol (FAA) and processed for scanning electron microscopy (SEM) and light microscopy (LM). For SEM, buds were dissected under a Wild Heerbrugg (M8) stereoscope and dehydrated through an ethanol series. Material was critical-point dried using a Tousimis® Autosamdri® 815B–Series A unit, and mounted on aluminium stubs using double-sided tape for coating with platinum in an Emitech K550 sputter coater. Samples were examined using a Hitachi cold-field emission SEM S4700 at 2 kV, and images were recorded digitally for subsequent manipulation. For LM, mature preanthetic floral buds were dehydrated through an ethanol series to 100 % ethanol, then taken through an ethanol–Histoclear® series to 100 % Histoclear® using a Leica TP 1010 processor. Buds were embedded in Sigma® Paraplast plus paraffin, sectioned using a Reichert-Jung 2040 microtome and stained in a Leica autostainer XL using safranin and alcian blue. Slides were mounted with DPX mounting medium. Additional floral buds were embedded in acrylic LR White resin, and sections (6 µm thick) were obtained using a Leica RM 2155 microtome and a tungsten carbide blade.
Inflorescences of Quillaja saponaria are organized in dichasial (thyrsoid) cymes with a terminal flower flanked by lateral buds subtended by their respective bracts (Fig. 2A). In young flowering branches, the terminal flower is surrounded by several floral buds which represent the terminal flowers of their respective branches (Fig. 3A). These incipient branches are subject to further dichasial branching later in development. Flowers are pentamerous, with an actinomorphic calyx and corolla, and bisexual (Figs 2A and 3A–B). However, some buds appear functionally male due to the rudimentary nature of their carpels. Sepals are valvate and connate (Fig. 3B). The calyx consists of one adaxial, two lateral and two abaxial sepals (Fig. 2A). Some buds with six or seven sepals and petals or a tetramerous/hexamerous gynoecium were occasionally found. The sepals are united to each other by cohesive hairs (Fig. 3C), and contain abundant tanniniferous cells, especially in the hypodermal region, but also scattered in the mesophyll. The sepal vascular bundles are often surrounded by single-celled crystal idioblasts containing druses. Mature petals are similar to each other, free, slightly acuminate, basally clawed and their margins irregularly lobed, reinforcing organ asymmetry (Fig. 3D). Although the corolla itself is homomorphic (i.e. all petals have the same shape) and the corolla is actinomorphic, each petal is not perfectly symmetric (Fig. 3D). Petal aestivation is contorted. The petals possess a papillate epidermis, particularly at the ventral side, enclosing a mesophyll with scattered tanniniferous cells. The androecium is diplostemonous, i.e. with equal number of sepal and petals arranged in two alternating whorls in episepalous and epipetalous position (Fig. 2A).
Diagram of dichasial partial inflorescence in Quillaja saponaria (A), with fused and alternipetalous carpels; and Suriana maritima (B), with free and antepetalous carpels. Hatched shapes indicate subtending bracts and bracteoles; black shapes indicate sepals; white shapes indicate petals; grey shapes indicate stamens. lf, lateral flower; n, nectary; X, staminode.
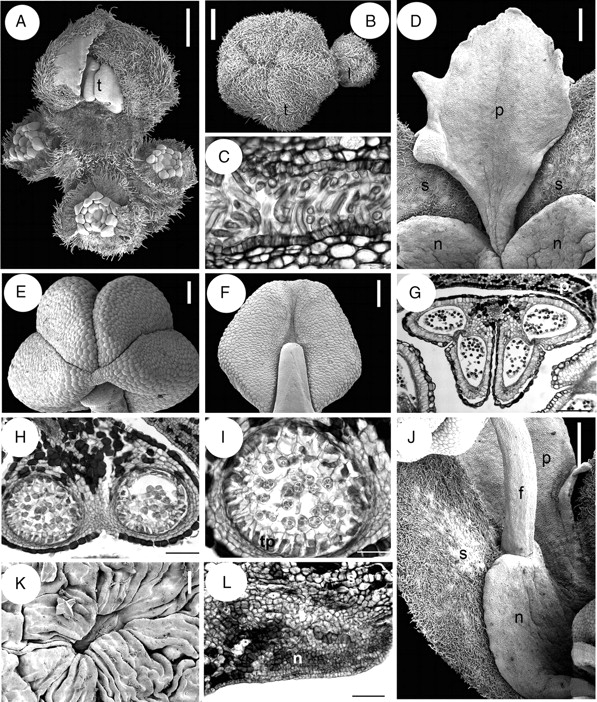
Quillaja saponaria, flower and inflorescence structure. A, B, D–F, J and K are SEM micrographs; C, G, H, I and L are transverse sections. (A) Young flowering branch with terminal bud and surrounding lateral buds. (B) Floral buds with hairy valvate calyx. (C) Sepals postgenitally united by cohesive hairs. (D) Petal at anthesis attached to a nectary sinus and alternating with sepals. (E, F) Top and dorsal view of anther. (G) Stamen transverse section with epidermis and connective with tanniniferous cells. (H) Theca and connective tissue. (I) Transverse section of sporangium with secretory parietal tapetum and tetrahedral tetrads. (J) Nectary lobe united with the opposite sepal and attached to a stamen. (K) A single stomata from a nectary. (L) Transverse section of sepal (above) and nectary (below). f, filament; l, lateral flower; n, nectary; p, petal; s, sepal; t, terminal flower; tp, tapetum. Scale bars: A, B, D, J = 0·5 mm; C, K = 20 µm; E, F = 250 µm; G = 200 µm; H, L = 100 µm, I = 50 µm.
Anthers are sagittate, tetrasporangiate, dorsifixed, versatile, caducous and introrse, with dehiscence lines intercepting the thecae division at the top of the anther; the dorsal furrow of the anther is shallow and the ventral one is deep (Fig. 3E–G). Within the anther, the tapetum is secretory and tetrads are tetrahedral (Fig. 3H, I). A receptacular nectary is fused to the sepals in mature flowers (Figs 2A and 3J); its surface is covered by several stomata (one of them shown in Fig. 3K) and it becomes very wet and conspicuous during anthesis. In transverse section of buds before anthesis, at the level of the anthers of the epipetalous stamens, the nectary–sepal fusion is evident by the conspicuous ventral glandular surface formed by eight to ten cell layers (Fig. 3L). The transition zone between the sepal and the nectary is occupied mainly by tannins and a conspicuous vascular bundle located in the middle. Antesepalous and antepetalous stamens are inserted at the nectary tips and sinuses, respectively (Figs 2A, 3J). The gynoecium consists of five alternipetalous carpels that are proximally fused, with the styles distally free from each other (Fig. 4A, B). Each carpel has a ventral suture readily discernible in mature flowers, extending from the ovary tip to the base. The proximal symplicate part of the gynoecium has a continuous unilayered pollen-tube transmitting tissue (hereafter abbreviated as PTT) covering both the ventral furrow of each carpel and the internal carpel–carpel union. The ovules are organized in two rows on marginal placenta (Fig. 4C). Stamens and gynoecium possess abundant tanniniferous tissue and some crystal idioblasts in the filaments, anther epidermis and connective (Fig. 3G–I), the dorsal side of the carpels (Fig. 4D) and style (Fig. 4E). At the distal part of the style below the stigmatic area, the PTT covers the ventral furrow, and is supplied by two to three vascular bundles (Fig. 4E). The stigmatic surface occupies the distal revolute part of the style and when receptive it forms a film (Fig. 4F). At the stigma, the ventral furrow is covered by long secretory cells supplied by abundant vasculature (Fig. 4G). Buds with underdeveloped gynoecia were found (Fig. 4H). The pedicel bears abundant unicellular thick-walled warty trichomes; parenchymatous ground tissue possesses abundant tanniniferous regions and cluster crystals (druses). Inflorescence axes, sepals and mature gynoecium are similarly covered by abundant warty trichomes (Figs 3A, D, J and 4A, B, I).
Quillaja saponaria, floral structure. A, B, F, H and I are SEM micrographs; C picture under stereoscope; D, E and G transverse sections. (A) Top view of gynoecium. (B) Distal and glabrous part of gynoecium. (C) Rows of ovules attached at the marginal side of a carpel; carpel wall partially removed. (D) Symplicate area of gynoecium in transverse section surrounded by layers of tanniniferous tissue. (E) Transverse section, style below stigmatic area displaying three main vascular bundles, and PTT covering ventral furrow. (F) Exposed stigmatic zone at anthesis. (G) Transverse section of style at stigmatic area level with secretory cells covering ventral furrow. (H) Fully developed anthers surrounding underdeveloped gynoecium. (I) Indumentum covering inflorescence axes, sepals and gynoecium, with warty ornamentation. c, carpel; o, ovule. Scale bars: A, C, H = 0·5 mm; B = 150 µm; D = 200 µm; E, G = 50 µm; F = 250 µm; I = 10 µm.
Initially, the floral apex is oval in outline but during sepal initiation it displays almost radial symmetry (Fig. 5A). Following sequential initiation of the bracteoles surrounding each floral bud (not shown), the calyx is initiated in a helical sequence, commencing at the abaxial side and culminating with the rising of the lateral sepals (Fig. 5A). Sepal initiation can follow a clockwise or anticlockwise sequence. By the time of sepal initiation in a terminal flower, the lateral buds emerge laterally (arrows in Fig. 5A). Corolla initiation takes place after sepal initiation and is relatively fast; it follows a helical pattern starting with the abaxial petal (Fig. 5B). The antesepalous stamen whorl is initiated before the antepetalous one (Fig. 5C, D). In some buds there was some overlap between initiation of later petal primordia and early stamen primordia (Fig. 5E). Although both whorls of stamens initiate unidirectionally, opposite primordia from the same whorl tend not to start simultaneously, and initially display dissimilar sizes (Fig. 5C, E, F). After androecium initiation, independent carpel primordia emerge simultaneously, alternating with the antepetalous stamen primordia and leaving the centre of the floral apex unoccupied (Fig. 5G).
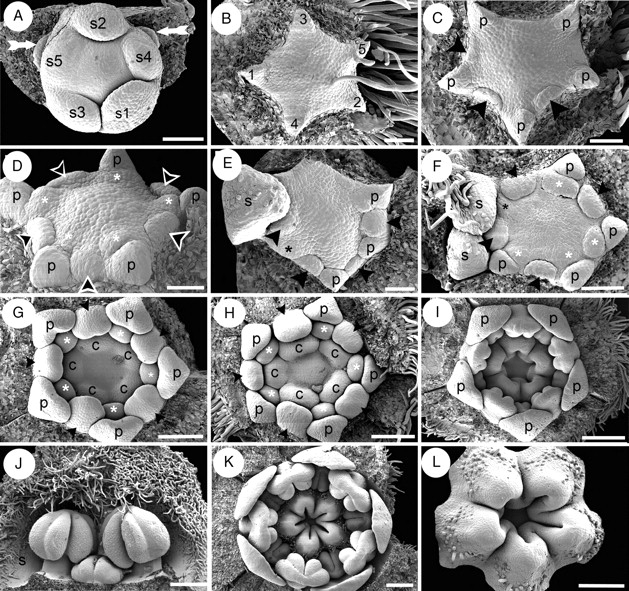
Quillaja saponaria, floral ontogeny. SEM micrographs. (A) Helical sepal initiation and two floral buds in the course of initiation (white arrows); subtending bracts of floral buds removed. (B) Helical initiation of petals indicated by numbers. (C) Unidirectional initiation of antesepalous stamen whorl (arrowheads) starting at the abaxial side; note that opposite stamen primordia do not develop simultaneously. (D) Floral bud after initiation of antesepalous stamens (black arrowheads) and antepetalous stamens (asterisks). (E) Hexamerous flower with unidirectional development of antesepalous stamen whorl (black arrowheads); adaxial petal primordium removed (upper side of the figure). Note the overlapping initiation of the last petal (black asterisk) and the stamens. (F) Floral bud with overlapping initiation of petals and stamens; adaxial petal (black asterisk) is underdeveloped with respect to stamens. Antesepalous (arrowheads) and antepetalous (white asterisks) stamen primordia are indicated; note that one abaxial antesepalous stamen is missing. (G) Independent and simultaneous initiation of carpels; antesepalous (black arrowheads) and antepetalous stamens (white asterisks) form two distinctive whorls. (H) Further development of gynoecium before lateral carpel fusion takes place. (I) Carpels start to fuse and form a continuous structure; the stamens are already differentiated into filament and anther. (J) Differentiated stamens from outer and inner androecium whorls; the outer members possess an enlarged filament. (K) Floral bud showing open gynoecium; ovule initiation is not evident at this stage, and petals remain distant from each other. (L) Carpels united at base and developing indumentum on their dorsal sides; ovules not yet evident. Abaxial floral side orientated to the bottom (A, C, E), to the right (F–I, K) and to the left (B). c, carpel; n, nectary; p, petal; s, sepal; s1–s5, order of initiation of sepal primordia. Scale bars: A, F = 100 µm; B–E = 50 µm; G, H, L = 150 µm; I, K = 200 µm; J = 250 µm.
By the time of stamen initiation, the sepals entirely cover the floral bud, exhibiting their characteristic indumentum and valvate aestivation. The petals, which enlarge in parallel with the stamens, are restricted to the corners of the pentagonal floral bud and become concave before the anther–filament differentiation (Fig. 5G, H). Although the antesepalous stamens possess longer filaments than the antepetalous ones during mid and late development, anther differentiation is simultaneous in both whorls (Fig. 5H–J). After independent initiation, carpels start to enlarge separately (Fig. 5H), but become basally fused (connate) during enlargement, forming a continuous structure (Fig. 5I, K, L). Ovule initiation was not observed prior to carpel closure. Placentation is marginal, and the ovules are anatropous and in two rows (Fig. 4C). The petals remain separated from each other during development.
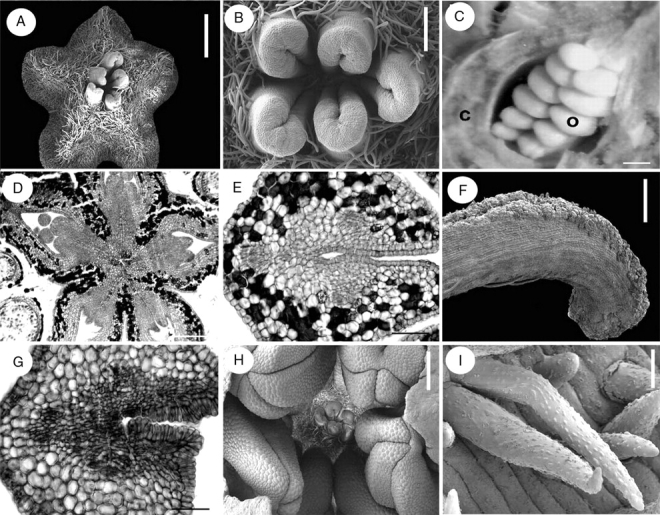
Inflorescences of Suriana maritima are dichasial cymes (Figs 2B and 6A). The inflorescence axis and the abaxial surface of the sepals are abundantly covered with multicellular glandular trichomes (Fig. 6A, B), whereas the mature carpels and stamens are covered proximally with longer unicellular hairs (Figs 6C, D). The warty hairs commonly observed in Quillaja saponaria are not present in Suriana maritima. The cortex of the pedicel bears jigsaw-like (interlocking) parenchymatous cells, scattered druses and mucilage inclusions surrounding vascular bundles (Fig. 6E). Flowers are pentamerous, actinomorphic and bisexual. However, in some individual buds (Ronse Decraene 1422) four carpels (Fig. 6F) or six stamens, one of them occupying the position of a carpel (not shown), were observed. Before sepal inception, the periphery of the floral base is surrounded by a multilayered region of tanniniferous cells, which is maintained at the dorsal side of the sepals. Sepals of Suriana maritima are quincuncial and fused at their base; there is one adaxial sepal, two laterals and two abaxial sepals (Fig. 2B). The sepals contain scattered druses surrounding vascular bundles or located at the ventral side (Fig. 6G–I). Petals are broad and initially free, later developing contort aestivation (Fig. 6J); their predominantly glabrous surfaces occasionally possess ornamented long hairs (Fig. 6K). The petal bases are slightly clawed and separated from each other (Fig. 6J). The androecium consists of an antesepalous whorl of fertile stamens and an antepetalous whorl of one to five elongated staminodes (Fig. 6C, D, F). The staminodes of different flowers do not occupy the same positions within the androecial outer whorl when they are less than five in number. The bases of the staminodes surround the petal base, forming a thick ring-like structure (Fig. 6C), although some thick petal bases lack associated staminodes (Fig. 6D). In transverse sections this ring-like structure contains small cells with prominent nuclei, but there is no clear evidence suggesting that they could be nectaries (Figs 6L, M and 7A). No nectary tissue was observed in flowers of Suriana maritima. Internally the staminodes consist of parenchyma with scattered druses; vascular tissue is not evident. The filaments, similarly as in petals and sepals, contain several druses (Fig. 7B–D). Anthers are X-shaped, tetrasporangiate, basifixed, versatile, caducous and introrse, with dehiscence lines intercepting the theca division at the top and bottom of the anther; the dorsal and ventral furrows of the anther are deep (Fig. 7E–G) (terminology follows Endress and Stumpf, 1991); they possess a thick epidermis with a prominent cuticle (Fig. 7H, I). The tapetum is secretory (Fig. 7I). The five antepetalous carpels form an apocarpous gynoecium (Fig. 7J). The carpels possess similar histology to the stamen filaments. Each carpel has an extended ventral furrow that closes prior to ovule inception. At the level of ovule inception, the furrow appears continuous with the ovule tissue (Fig. 7K). Placentation is basal-marginal (Fig. 7K). The ovule integument possesses ventral projections that indicate an obturator (Fig. 8A). Inside each carpel there are two ovules surrounded by mucilage (Fig. 8B). At the level of the top of the ovules, the carpel shows a gynobasic condition (Fig. 7J, 8B). Between the ovary and anther levels of a bud before anthesis, the styles are kidney-shaped in cross-section, and their ventral furrow is a small canal surrounded by vascular tissue; the canal communicates with the exterior (Fig. 8C). At the anther level, the canal is closed, leaving it isolated from the exterior at this level (Fig. 8D). Below the stigma level, the canal is larger in diameter, filled with mucilage and surrounded by the PTT (Fig. 8E, F). At the apex of each style, a stigmatic papillate surface develops (Fig. 8G). Hairs covering the inflorescence axes consist of a multicellular glandular head supported by a three- or four-celled stalk (Figs 6B and 8H).
Suriana maritima flower and inflorescence morphology. A–D, F, J and K are SEM micrographs; E, G–I, L and M are transverse sections. (A) Lateral view of a dichasial cyme; the terminal flower is surrounded by two partial inflorescences, each repeating the dichasial cyme structure. (B) Glandular multicellular hair growing on sepal. (C) Dorsal view of a mature hairy staminode with extended ring-shaped tissue surrounding petal base (black arrow, petal detached). (D) Lateral view of mature androecium and gynoecium; thickened petal bases with and without associated staminodes (black arrows). (E) Pedicel. (F) Polar view of floral bud with missing carpel and four staminodes (asterisks). (G) Section of a sepal showing a single stomata in the abaxial (ventral) epidermis. (H) Sepal; ventral side towards the upper side of the figure. (I) Sepal vascular bundle surrounded by tissue with oxalate druses. (J) Lateral view of preanthetic bud with contorted petal aestivation. (K) Ornamented hair from petal. (L) Floral receptacle with petal and staminodial tissue differentiating. Petals (their vascular bundles indicated by arrows) surrounded by the base of the staminodes (white asterisks). Stamens are indicated by black asterisks. (M) Petal bases surrounded by staminode base; arrowheads indicate petal vascular tissue. c, carpel; p, petal; r, receptacle; s, sepal; sy, style; t, terminal flower. Scale bars: A, C = 200 µm; B, G = 20 µm; D, L = 1 mm; E, M =100 µm; F = 150 µm; H = 50 µm; I = 2 µm; J = 0·5 mm; K = 10 µm.
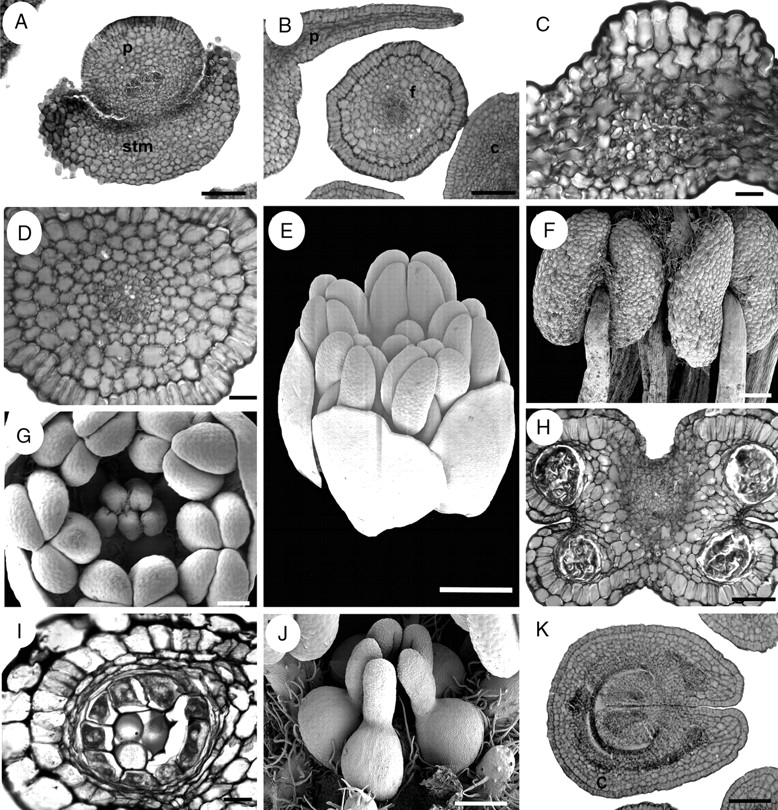
Suriana maritima, floral structure. E–G and J are SEM micrographs; A–D, H, I and K are transverse sections. (A) Petal surrounded by staminode base. (B) Petal and filament. (C) Petal, dorsal side at the upper side of the figure. (D) Filament. (E) Oblique view of bud at mid-development. (F) Dorsal view of basifixed, mature, X-shaped anthers. (G) Polar view of floral bud showing gynoecium and androecium during mid-development. (H) Transverse section of an anther. (I) Sporangium with parietal tapetum. (J) Lateral view of apocarpous, pentamerous gynoecium during mid-development. (K) Proximal side of carpel showing marginal placentation of ovules. c, carpel; f, filament, p, petal; stm, staminode. Scale bars: A, B, H, K = 100 µm; C = 10 µm; D, I = 20 µm; E = 0·5 mm; F, G, J = 200 µm.
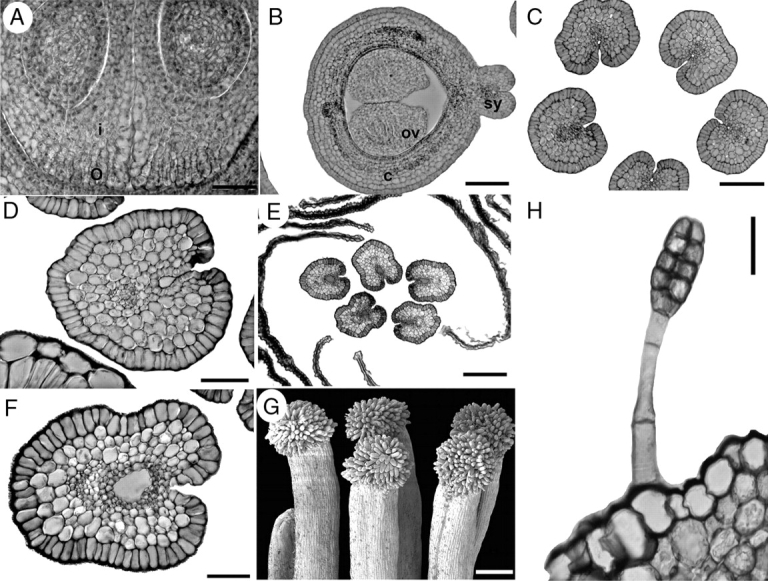
Suriana maritima, floral structure. G is an SEM micrograph; A–F and H are sections. (A) Transverse section of a carpel displaying the integument and obturator projections. (B) Transverse section of the distal part of ovules; note the carpel filled with mucilage. (C) Transverse section of styles at the level below the anthers; stylar canal is isolated from the exterior at the ventral side. (D) Style at the level of the anthers, with stylar canal filled with mucilage. (E, F) Styles above anther level displaying closed stylar canal surrounded by PTT and filled with mucilage. (G) Side view of group of mature papillate stigmas. (H) Glandular hair on sepal. c, carpel; i, integument; o, obturator; ov, ovule; sy, style. Scale bars: A, D = 50 µm; B, C = 100 µm; E, G = 200 µm; F, H = 20 µm.
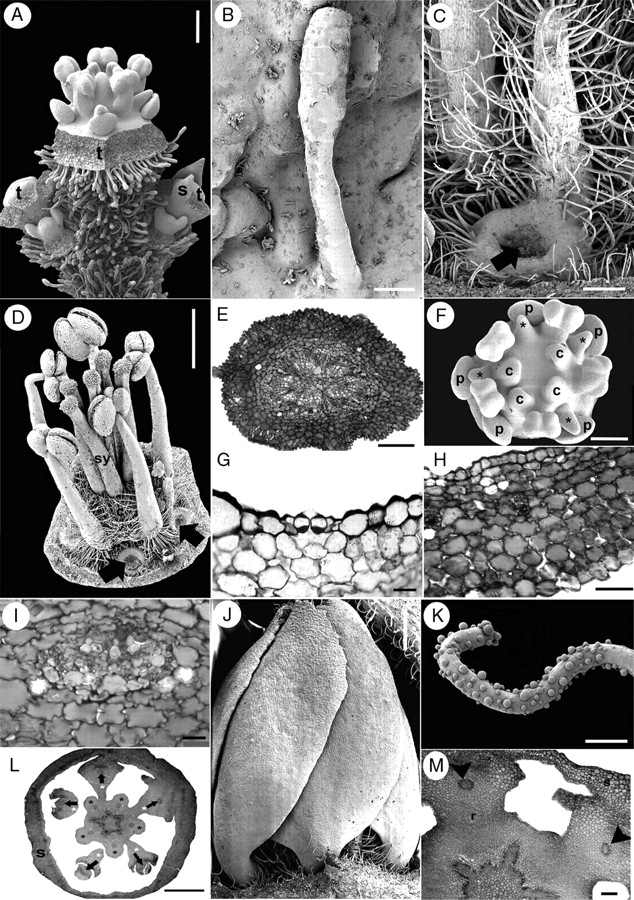
The lateral flowers of the first order develop in a basipetal sequence in young flowering branches (Fig. 9A). An ovoid floral bud develops in the axil of the subtending bract and soon afterwards, paired bracteoles are initiated sequentially (Fig. 9B, C). In each inflorescence branch, the lateral floral buds become evident during perianth initiation of the terminal flower (Fig. 9C). Sepal and petal initiation proceeds helically, as in Quillaja saponaria (Fig. 9A, B, D–G). However, androecium initiation is different; in Suriana maritima the antesepalous stamens emerge simultaneously after helical petal initiation (Fig. 9G). Soon the antepetalous stamens are initiated simultaneously and centrifugally with respect to the antesepalous stamen primordia (Fig. 9H). There are usually fewer than five antepetalous stamens. The antepetalous carpels are initiated simultaneously after the staminodial whorl (Fig. 9H, I). Following carpel initiation, the antepetalous staminodes start to differ morphologically from the fertile stamens, and the floral apex enlarges forming a short internode (gynophore-like structure) between the androecium and the carpel primordia (Fig. 9I, J). Petals remain free and separate from each other during floral enlargement. The antesepalous stamens and the carpels grow rapidly with respect to the petals. The staminodes enlarge, acquire hairs at their proximal side and extend their basal part around the petal base (Fig. 9K). The carpels grow homogeneously and remain independent from each other (Fig. 7J).
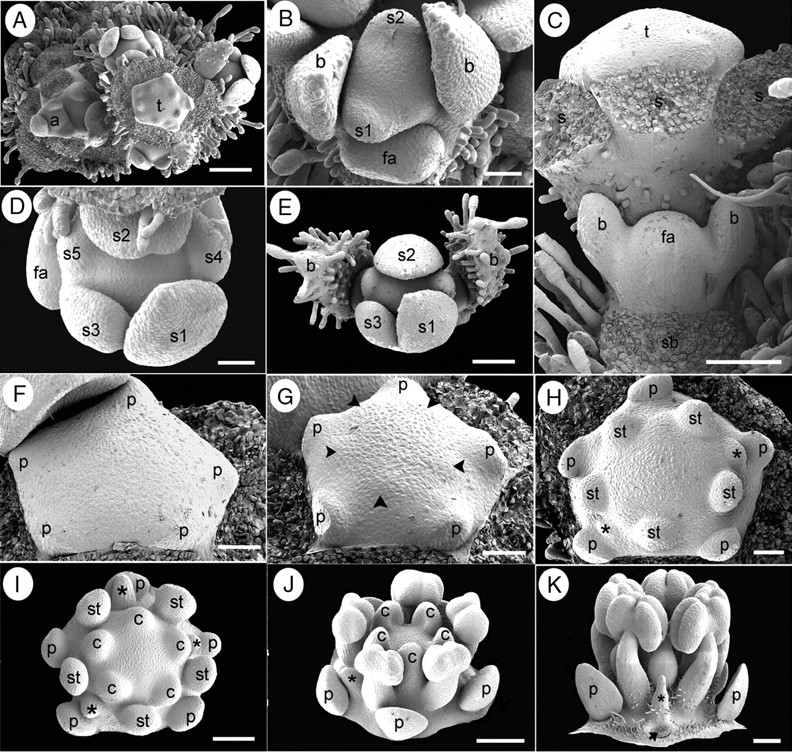
Suriana maritima floral ontogeny. SEM micrographs. (A) Young flowering branch with lateral floral bud initiation. (B) Polar view of floral bud during sepal initiation flanked by a younger floral apex (bottom). (C) Lateral view of a partial inflorescence with lateral floral apex immediately after bracteole initiation; sepals of terminal flower were removed. (D) Polar view of floral bud after helical sepal initiation. (E) Polar view of a young dichasial partial florescence; terminal flower surrounded by bracts subtending young floral buds. (F) Floral bud during petal initiation. (G) Floral bud during simultaneous initiation of antesepalous stamen primordia (arrowheads). (H) Floral bud with antesepalous stamen primordia alternating with two staminode primordia (asterisks). (I) Floral bud after carpel initiation; carpels are initiated opposite staminodes (asterisks) and petals. (J) Oblique view of floral bud with basifixed anthers, staminode (asterisk) and concave carpels at the top of enlarged gynophore. (K) Lateral view of floral bud with stamens, staminode (asterisk) and floral base becoming hairy; petal base is pointed (arrow). a, branch apex; b, bracteoles; c, carpel; fa, floral apex; p, petal; s, sepal; s1–s5, order of sepal initiation; sb, subtending bract position; st, stamen; t, terminal flower. Scale bars: A = 200 µm; B, D, F–H = 50 µm; C, E, I = 100 µm; J, K = 150 µm.
Quillajaceae and Surianaceae are relatively species-poor lineages compared with Leguminosae and Polygalaceae, and their floral morphology and development are unusual within Fabales. However, in a broader comparative context within eurosids I, their characters could represent a mixture of symplesiomorphies and autapomorphies, although this has yet to be tested in a morphological cladistic analysis. Molecular data currently offer five alternative hypotheses of interfamilial relationships in Fabales (Fig. 1; M. A. Bello et al., unpubl. res.). Of these, two indicate that Quillajaceae and Surianaceae are sister families (Fig. 1B,E). Floral traits shared by Quillajaceae and Surianaceae include inflorescences organized as dichasial cymes, predominantly actinomorphic floral symmetry, median position of the adaxial sepal and abaxial petal, contort petal aestivation, free petals and stamens, similar petal shape, dorsifixed and longitudinally dehiscent anthers, and five carpels (or fewer in some Surianaceae). However, many of these characters are not exclusive to these two families, and also occur in other eurosids I and/or in other Fabales. For example, the determinate inflorescences of Quillajaceae and Surianaceae (Fig. 2A, B) contrast with the predominantly indeterminate racemose inflorescences of Leguminosae and Polygalaceae. However, cymes are widely distributed in several orders of eurosids I, such as Cucurbitales (Matthews and Endress, 2004), Fagales (Rozefelds and Drinnan, 2002; Li et al., 2005), Rosales (Medan and Hilger, 1992; Okamoto et al., 1992; Evans and Dickinson, 1999a, b; Ronse de Craene and Miller, 2004; Evans and Dickinson, 2005), Oxalidales (Matthews and Endress, 2002), Zygophyllales (Sheahan, 2007; Simpson, 2007) and some Malpighiales (Dahlgren, 1988; Ronse de Craene and Smets, 1991; Jäger-Zürn and Mathew, 2002; Merino Sutter and Endress, 2003). This suggests that either cymose inflorescences were acquired several times independently, or they represent a homologous feature with several losses, at least at the level of eurosids I. On the other hand, this character is not exclusive to Quillajaceae and Surianaceae within Fabales. Within Leguminosae, cymes are present in Dialum guianense, Gleditsia triacanthos, Poeppigia procera and Psoralea pinnata (Weberling, 1989; Tucker, 1987, 1991, 1998). Within Polygalaceae, they occur in some species of Xanthophyllum (van der Meijden, 1982).
Quillajaceae and Surianaceae also share predominantly actinomorphic floral symmetry, in contrast to most other Fabales. However, this feature is similarly widely distributed in several Cucurbitales, Fagales, Rosales, Oxalidales, Zygophyllales and Malphigiales (Matthews et al., 2001; Matthews and Endress, 2002, 2004, 2005) as well as in some Polygalaceae and several unrelated Leguminosae (i.e. Mimosoideae and some Caesalpinioideae). This distribution suggests that actinomorphy is plesiomorphic in Fabales. However, it is important to review the transition to zygomorphy in conjunction with changes in inflorescence architecture and loss of terminal flowers in Fabales, because floral symmetry is closely associated with relative position of flowers within an inflorescence (see Endress, 1999, for discussion of positional monosymmetry). Cymose members of Leguminosae tend to possess an actinomorphic perianth, but flowers are zygomorphic in Dialum (Tucker, 1998) and Psoralea pinnata (Tucker and Stirton, 1991). Similarly, in Xanthophyllum (Polygalaceae) zygomorphic non-papilionate flowers can be organized in cyme-like branches (e.g. in X. octandrum; van der Meijden, 1982, fig. 22). A simultaneous systematic approach to these traits could shed further light on their distribution in different lineages of Fabales.
A median adaxial position for one of the sepals (and thus abaxial for a petal) is characteristic for Quillajaceae and Surianaceae, and also occurs in Polygalaceae and some Leguminosae, although legumes most commonly exhibit the reverse orientation: an abaxial sepal and an adaxial petal (Tucker, 1987). A similar arrangement (adaxial sepal and abaxial petal) occurs in Oxalidaceae, Cucurbitaceae, Rosaceae, Tremandraceae and Zygophyllaceae (Eichler, 1875). On the other hand, antesepalous placement of the pentamerous gynoecium, ventral marginal placentation and two rows of ovules, plus the presence of a diplostemonous androecium, represent a unique set of characteristics for Quillaja. In Coriaria myrtifolia (Coriariaceae, Cucurbitales) the gynoecium is similarly pentamerous, antesepalous and surrounded by a diplostemonous androecium, but each locule is occupied by a single ovule with apical and median placentation, and stigma and style morphology differ considerably from that of Quillaja (see Matthews and Endress, 2004, figs 9 and 10). Similarly, Gillenia trifoliata (Rosaceae) possesses five antesepalous carpels, but in this species there are two ovules and a polyandrous androecium (cf. Eichler, 1875, fig. 217B). In Surianaceae, the apocarpous, antepetalous and predominantly pentamerous gynoecium, plus the presence of an obdiplostemonous androecium, seem to be autapomorpic. Although Quillajaceae and Surianaceae can be differentiated from other eurosids and Fabales by their reproductive whorls, there is little evidence in these organs for a sister relationship between them.
In Quillaja and Suriana the calyx is initiated helically, starting at the abaxial side of the flower, as in Polygalaceae (M. A. Bello, unpubl. res.) and several legume genera (Tucker, 1992, 2000a, b, 2001a, b, 2002a, b, 2003; Endress, 1994). Quillaja possess a valvate calyx with sepals united postgenitally by cohesive hairs (Fig. 3B, C), and in Suriana the calyx is imbricate with the lobes postgenitally fused at the base. The relative position of the sepals after calyx initiation is similar in both genera. In Delonix (Caesalpinioideae), the valvate calyx has been interpreted as derived from an initial imbricate quincuncial arrangement by thickening and broadening of the sepals (Endress, 1994); a similar situation occurs in Quillaja (Fig. 3B). If this interpretation proves correct, all families of Fabales show helical initiation of the calyx. In contrast to Gutzwiller's (1961) report that the stamens initiate before the corolla in Suriana, we found that the stamens are initiated after the petals in both Suriana and Quillaja, continuing the helical sequence of initiation imposed by the calyx. This feature also occurs in the legume genera Amherstia, Cassia, Gleditsia, Dimorphandra, Erythrophleum, Schotia, Senna and Tamarindus (Tucker and Douglas, 1994) and similar patterns are found in Polygalaceae.
Despite similar perianth initiation in Quillaja and Suriana, these genera differ considerably in stamen initiation and development. In Quillaja initiation is unidirectional and asymmetric (Fig. 5C–F), and in Suriana it is simultaneous in each whorl (Fig. 9G–H; see also Gutzwiller, 1961). In both genera, the antesepalous stamens are initiated before the antepetalous whorl, but in Suriana the antesepalous stamens are closer to the floral centre than the antepetalous staminodial ones, thus forming an obdiplostemonous androecium (Fig. 9I, K). Furthermore, Suriana has an antepetalous carpel position and reduced antepetalous stamens, a feature almost exclusively associated with obdiplostemony (Eckert, 1966; Ronse de Craene and Smets, 1998). The diplostemonous condition, exhibited in Fabales by Quillaja and some Leguminosae and Polygalaceae, is characterized by alternating stamen and carpel whorls when isomerous, an outer antesepalous stamen whorl, and an inner antepetalous and centripetally initiated stamen whorl (Ronse de Craene and Smets, 1995). Diplostemony is widely distributed in angiosperms; in several families it is considered the plesiomorphic condition (Ronse de Craene and Smets, 1995). Within eurosids I, obdiplostemony is recorded in the orders Cucurbitales, Celastrales, Oxalidales and Zygophyllales (Ronse de Craene and Smets, 1995; Matthews and Endress, 2002, 2004, 2005; Sheahan, 2007). We predict that a broad comparative developmental study of obdiplostemonous androecia in eurosids I, evaluated in a phylogenetic context, will display different developmental pathways for the acquisition of this character (for variation in obdiplostemony see Ronse de Craene and Smets, 1995).
The androecium can strongly influence the number and position of the carpels (Ronse de Craene and Smets, 1991, 1994, 1998). Thus, in Quillaja and Suriana, differential organization and later development of the gynoecium is probably influenced by differences in the androecium (Figs 1, 5G–I, K, L and 9I, J). Other morphological differences between flowers of Surianaceae and Quillajaceae include the presence of a gynophore in some Surianaceae (Recchia: Loesener and Solereder, 1905; Cronquist, 1981; Crayn et al., 1995) and Suriana (Figs 6A and 9I, J), mucilage-filled stylar canal and capitate stigmas in Surianaceae (Fig. 8E–G), and a decurrent stigma and conspicuous lobed nectary in Quillaja. The dissimilar development and morphology of the reproductive whorls of Quillaja and Suriana do not support a sister-group relationship between the two families. If ‘resolving synapomorphies is not a problem if relevant ontogenetic information is available’ (Nelson, 1978: 325), we expect our ontogenetic characters to be congruent with hypotheses of relationships. If phylogenies based on molecular data depict the actual systematic relationships of the angiosperm families, the conflict with developmental data suggests differential degrees of constraint and changes in developmental characters along the history of different lineages that merit further exploration. It is also possible that floral ontogeny in Fabales is insufficiently informative at the interfamilial level, perhaps due to a rapid lineage origin accompanied by profound changes in formerly synapomorphic floral traits. Some comparative studies of floral ontogeny examining interfamilial relationships have found contrasting evidence, or no evidence, for hypotheses derived from molecular analyses (e.g. Caris et al., 2000, 2002; Ronse de Craene et al., 2000; Caris and Smets, 2004; Erbar et al., 2005). However, sequence-based results have found support from developmental surveys (e.g. Bernhard and Endress, 1999; Ronse de Craene and Smets, 2001).
It is currently impossible to propose a floral character as a potential synapomorphy for a sister-pairing of Quillaja and Suriana (Fig. 1B and E), because the traits they share are also present either in other families of eurosids I, or in other Fabales. On the other hand, several characters present in Quillajaceae also occur in some legumes. For example, the common pentamerous ground plan of legume flowers includes ten stamens in two whorls with members alternating radially (Tucker, 1987), and the unidirectional/asymmetrical initiation of the androecium observed in Quillaja and several Leguminosae (see, for example, Tucker, 1987, 1999) is remarkable. Contrary to other Fabales, carpel position is antesepalous in both Quillaja and several legumes, and placentation is ventral-marginal, forming two rows of ovules (Fig. 4H). Interestingly, van Heel (1993) interpreted the occurrence of five antesepalous carpels in Archidendron lucyi (Mimosoideae) as the plesiomorphic condition for Leguminosae, which would suggest that the unicarpellate condition in legumes is derived from a pentamerous gynoecium like that of Quillaja. However, this hypothesis is not supported by recent phylogenies of Leguminosae, in which Mimosoideae occupy a derived clade within the unicarpellate Papilionoideae. Eichler (1875) noted a similarity between legume pods and the dehiscence pattern of individual follicles in Quillaja. Despite their similarities, we cannot readily interpret these characters as synapomorphies for a potential relationship between Quillajaceae and Leguminosae because they could equally represent either plesiomorphic characters that were lost or changed in Surianaceae and Polygalaceae, or even independent acquisitions. Furthermore, their distribution has not yet been tested cladistically, and the clade (Quillajaceae + Leguminosae) is not included among the possible molecular-based hypotheses available for Fabales (Fig. 1). A recent palynological study of Fabales suggested that pollen morphology of Quillajaceae has more in common with that of Leguminosae (Cercideae) and Surianaceae (especially Cadellia) than with Polygalaceae (Claxton et al., 2005). Floral development and morphology of Polygalaceae are unusual with respect to other Fabales (M. A. Bello, unpubl. res.), but several floral characters shared by Leguminosae, Surianaceae and Quillajaceae also occur in eurosids I (e.g. the presence of carpels without a congenitally fused base). Thus, this initial approach to the floral development of the species-poor families Quillajaceae and Surianaceae cannot provide answers to the macrosystematics of Fabales without a more rigorous cladistic analysis that clarifies whether characters are plesiomorphic or homoplastic.
It is tempting to speculate that the contrasting species number among families of Fabales (fewer than ten in Quillajaceae and Surianaceae versus approx. 20 000 in Leguminosae and 1000 in Polygalaceae) is associated with differences in floral symmetry; flowers of Quillajaceae and Surianaceae are actinomorphic, but those of Leguminosae and Polygalaceae display different types of zygomorphy (monosymmetry), including the distinctive papilionoid floral shape. Additionally, several actinomorphic genera of both Leguminosae (e.g. Holocalyx, Lecointea, the Umtiza clade members; Lewis et al., 2005) and Polygalaceae (e.g. Balgoya, Barnhartia, Diclidanthera; Eriksen, 1993) exhibit a relatively low species number. In other angiosperm groups with predominantly zygomorphic flowers, such as Lamiales s.l., Leguminosae, Orchidaceae and Zingiberales, monosymmetry is interpreted as a key innovation that was accompanied by an explosive radiation (Endress, 1999). Supporting this idea, and based on statistical sister-group comparisons, Sargent (2004) concluded that clades with zygomorphic corollas are more species-rich than their actinomorphic sister groups, and suggested an association between monosymmetry and speciation. However, a further study of Kay et al. (2006) found that the influence of zygomorphy on diversification is variable. In Fabales floral symmetry ranges from actinomorphic in all whorls (Quillaja), to asymmetric/monosymmetric in some whorls (e.g. the androecium of Suriana, the corolla in some caesalpinioids), or strongly zygomorphic in the entire flower (most papilionoids and several Polygalaceae). Additionally, actinomorphy could have originated several times independently in the order, as a plesiomorphic trait in Quillajaceae and Surianaceae and as a derived condition in legumes such as Aldina, Amphimas, Baphiopsis, Cadia and Lecointea (Pennington et al., 2000). Whorl symmetry can change through time (Tucker, 1987) and between close relatives in the order, so testing a correlation between species richness and particular symmetry patterns must involve examination of the development of key floral morphologies within reasonably well-sampled clades. Additionally, it would require analysis of what key innovation represents in this case, either an unreversed synapomorphy that may or may not be involved in branching rate (Sanderson and Donoghue, 1994; Ree, 2005), or a homoplasious trait of derived clades radiating independently (Claßen-Bockoff et al., 2004). Interestingly, phylogenetic studies including floral characters in Caesalpinioideae found zygomorphy to be a feature of a multitude of homoplasious morphs (Bruneau et al., 2005). If a positive correlation between species richness and those independently derived morphs is demonstrated, zygomorphy could be a key innovation promoting increased branching rate in independent lineages in Fabales.
Different surveys across several angiosperm phylogenies suggest that species diversification is positively affected by animal pollination and the presence of floral nectar spurs (von Hagen and Kadereit, 2003), and negatively affected by a dioecious sexual system (Heilbuth, 2000). Unfortunately, there is currently insufficient information on reproductive biology in Fabales to evaluate potential relationships between sexual systems and the observed diversification of the families. Research has been carried out in this area in several Caesalpinioideae, in which there are diverse pollination–sexual systems, including unisexuality (in Arcoa, Ceratonia, Gymnocladus, Tetrapterocarpon), polygamo-dioecy in Gleditsia, potentially cryptic dioecy in some Caesalpinioideae (e.g. Lewis et al., 2000) and anemophily in Ateleia (Janzen, 1989). In Surianaceae, wind pollination was documented for Stylobasium by Prance (1965). The unisexuality observed in some flowers of Quillaja, in which the gynoecium is aborted during mid-development, could be an interesting feature affecting reproductive biology in the family.
MAB was funded by Alßan, the European Union Programme of High Level Scholarships for Latin America (E04D040438CO), ORSAS, Overseas Research Students Award Scheme UK (2005033009), and COLCIENCIAS, Colombia (project code 11010517595) during different stages of this study. We thank Peter Endress (University of Zurich) and Shirley Tucker (University of California-Santa Barbara) for the valuable comments they made to this manuscript. We acknowledge Favio González (Universidad Nacional de Colombia) for helpful discussion, and Louis Ronse de Craene (Royal Botanic Gardens Edinburgh) and F. Zapata (University of Missouri, St Louis) for facilitating use of their collections. Chrissie Prychid (Royal Botanic Gardens, Kew) provided invaluable laboratory support.